Manufacturing facilities across the pharmaceutical, food, and medical device industries face constant pressure to maintain regulatory compliance while ensuring product quality and safety. The FDA's Current Good Manufacturing Practice (cGMP) regulations establish the foundation for quality pharmaceuticals through proper design, monitoring, and control of manufacturing processes and facilities. A poster of good manufacturing practices serves as a constant visual reminder of these critical standards, reinforcing compliance culture throughout your operations.
This comprehensive guide explores how implementing GMP compliance poster strategies alongside robust quality management systems transforms regulatory requirements into operational excellence that protects both consumers and your business reputation.
Understanding Good Manufacturing Practices and Visual Compliance Tools
Good Manufacturing Practices represent systematic approaches to ensuring manufactured products meet consistent quality standards throughout production. GMP stands for Good Manufacturing Practices, a system that ensures manufactured products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards.
Visual compliance tools like GMP compliance posters serve essential functions in manufacturing environments by providing constant reference points for critical procedures and principles. These displays reinforce training, remind personnel of key requirements, and demonstrate commitment to quality standards during regulatory inspections.
Good Manufacturing Practice guidelines guide manufacturing, testing, and quality assurance to ensure that manufactured products are safe for human consumption or use. The visual nature of the poster on good manufacturing practices helps translate complex regulatory requirements into digestible, actionable information that personnel can reference throughout their workday.
Many manufacturing facilities implementing visual compliance strategies report improved adherence to procedures and reduced non-conformances when combined with comprehensive quality systems. The constant visual reinforcement helps embed quality culture within operational DNA, transforming compliance from a burdensome obligation into standard practice.
BPRHub's comprehensive Quality, Compliance, and Governance platform complements visual compliance tools by digitizing good manufacturing practices procedures and maintaining real-time documentation that supports both poster displays and electronic systems.
Elevate your compliance game, implement the 10 GMP principles seamlessly with BPRHub’s all-in-one platform.
📍 Book a Demo
📧 hello@bprhub.com
The 10 Core GMP Principles for Manufacturing Excellence
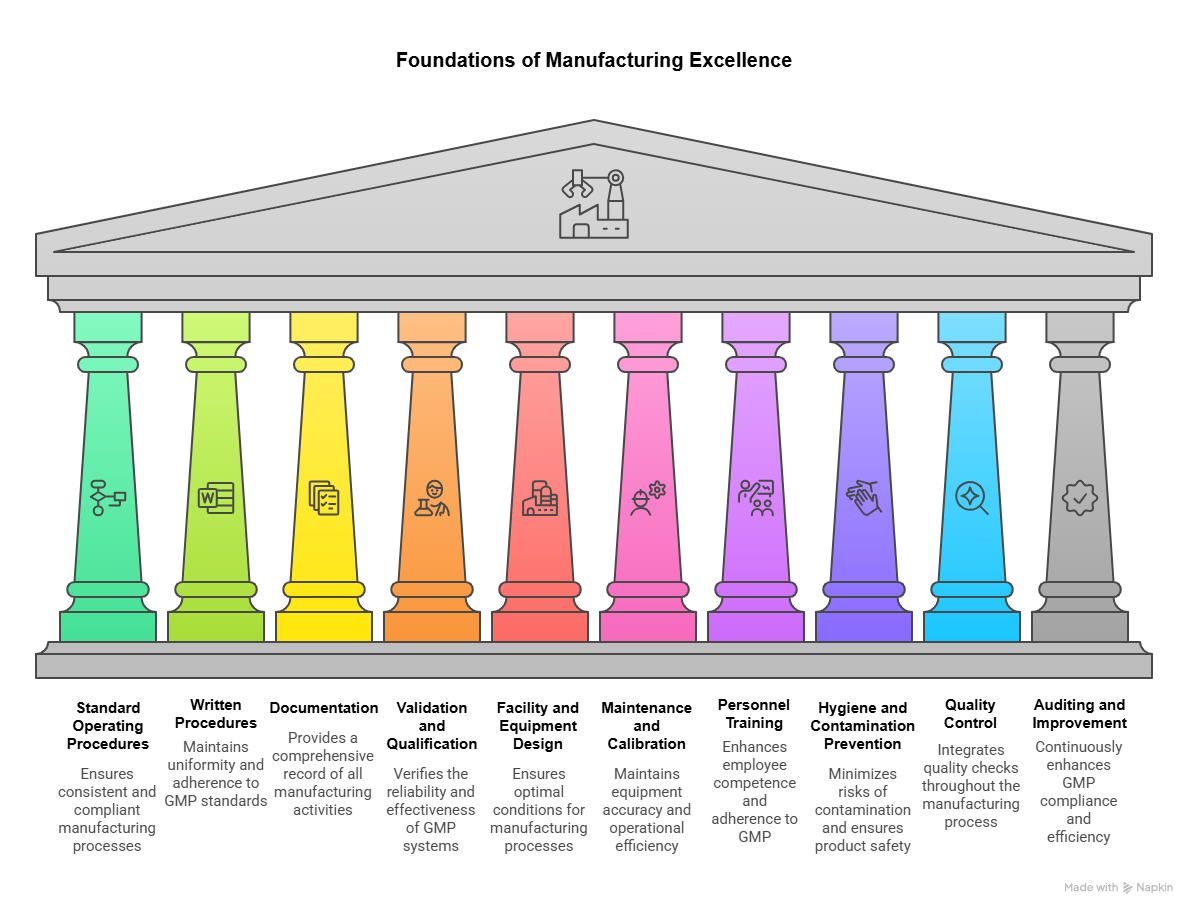
The 10 GMP principles cover all aspects of production, from the starting materials, premises, and equipment to the training and personal hygiene of staff. Understanding these principles provides the foundation for creating effective poster good manufacturing practices displays that communicate essential requirements.
Standard Operating Procedures for GMP Compliance
Detailed written procedures are essential for each process that could affect the quality of the finished product, and there must be systems to provide documented proof that correct procedures are consistently followed at each step. SOPs establish consistency across manufacturing operations while providing clear guidance for personnel performing critical tasks.
Effective GMP compliance poster displays should highlight the importance of following documented procedures and reference where personnel can access current SOPs. Visual reminders reinforce the critical nature of procedural compliance while supporting ISO 9001 good documentation control requirements.
Following Written GMP Procedures Consistently
Creating procedures without ensuring consistent adherence undermines quality systems. Personnel must understand that deviations from approved procedures require documentation and investigation before implementation.
Poster good manufacturing practices displays can reinforce this principle through visual cues that remind personnel to consult procedures before beginning tasks and document any deviations immediately. This constant reinforcement helps prevent procedural drift that compromises product quality.
Complete Documentation of GMP Manufacturing Activities
Incorporating quality directly into all production phases requires putting clearly defined controls in place and preserving accurate, timely records. Comprehensive documentation creates accountability while providing evidence of compliance during regulatory inspections.
Manufacturing documentation extends beyond batch records to include equipment maintenance, personnel training, calibration records, and deviation investigations. ISO 9001 quality record retention procedures ensure proper documentation management that supports both GMP compliance and business continuity.
Validation and Qualification in GMP Systems
Changes to the manufacturing process must be properly reviewed, validated, and documented to ensure the quality of the product isn't compromised. Validation confirms that processes consistently produce expected results, while qualification ensures equipment functions according to specifications.
GMP compliance poster displays should remind personnel that validated processes must not be modified without proper change control procedures. This visual reinforcement supports systematic approaches to process improvement while maintaining product quality integrity.
GMP Facilities and Equipment Design Requirements
This principle emphasizes the value of incorporating productivity, quality, and employee safety into the design and development of buildings and equipment, with materials, goods, and components separated to avoid confusion, mix-ups, and errors. Proper facility design minimizes contamination risks while supporting efficient workflow.
Equipment design considerations include materials of construction, cleaning accessibility, and integration with quality systems. Manufacturing facilities should clearly delineate production areas and implement controls that prevent cross-contamination between different products or production stages.
Regular Maintenance and Calibration for GMP
This principle involves maintaining a proper schedule that prevents equipment breakdown, reduces risks associated with product contamination, and ensures that facilities or equipment remain in validated states. ISO 9001 quality management preventive maintenance programs support this requirement through systematic scheduling and documentation.
Poster good manufacturing practices displays can highlight upcoming maintenance activities and remind personnel to report equipment malfunctions immediately. This proactive approach prevents quality issues while extending equipment lifespan.
Personnel Training and Competence in GMP
All employees are expected to strictly adhere to manufacturing processes and regulations, and current GMP training must be undertaken by all employees to fully understand their roles and responsibilities. ISO 9001 competence training requirements align closely with GMP expectations for personnel qualification.
Manufacturing organizations should implement comprehensive training programs covering both general GMP principles and job-specific requirements. GMP compliance posters display and reinforce training concepts while serving as quick reference guides for daily operations.
Personal Hygiene and Contamination Prevention
Sanitation and hygiene are vital in every aspect of the manufacturing process, covering anything that can cause contamination, such as personnel, premises, equipment, containers, and production materials. Strict hygiene protocols prevent product contamination that could compromise safety and efficacy.
Posters displaying good manufacturing practices are positioned near gowning areas and production entrances, reminding personnel of hygiene requirements before entering manufacturing spaces. Visual reinforcement complements training while maintaining contamination control culture.
Quality Control Integration in GMP
Quality control should be incorporated directly into all production phases by putting clearly defined controls in place, with good manufacturing, packing, labeling, distribution, and marketing all examples of quality control touchpoints. Quality cannot be tested into products; it must be built into manufacturing processes.
Manufacturing quality control extends beyond final product testing to include in-process controls, environmental monitoring, and raw material verification. Risk assessment in manufacturing helps identify critical control points requiring enhanced oversight.
Regular Auditing and GMP Continuous Improvement
Conducting regular audits represents the only way to identify how successfully GMP is applied and to undertake periodic audits to evaluate the efficacy of GMP compliance. Systematic auditing identifies improvement opportunities while demonstrating regulatory compliance.
Internal audit programs should assess adherence to all GMP principles while identifying systemic issues requiring corrective action. GMP compliance poster displays can communicate audit schedules and findings, reinforcing accountability culture throughout organizations.

The 5 Ps of GMP: Framework for Manufacturing Excellence
The "5 Ps" of GMP, People, Premises, Processes, Products, and Procedures, serve as a practical framework for understanding and implementing GMP requirements. According to the World Health Organization, Good Manufacturing Practice ensures that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use.
People: Foundation of GMP Quality Systems
GMP compliance starts with qualified, trained personnel who understand both general GMP principles and their specific roles, with ongoing training keeping staff up-to-date on industry standards and regulatory changes. ISO 9001 job description roles, responsibilities, and clarify expectations while supporting competency development.
Manufacturing personnel represent the most critical component of quality systems. Poster good manufacturing practices displays reinforce personnel responsibilities while communicating organizational commitment to quality culture.
Premises: GMP Facility Design and Maintenance
GMP-compliant facilities are designed to minimize contamination risks, with defined zones for each production activity and environmental controls like temperature, humidity, and air quality closely regulated. Proper facility design supports efficient operations while maintaining product integrity.
Processes: Validated GMP Manufacturing Operations
Processes must be validated and controlled, with manufacturers ensuring that primary materials, including raw products and other components, have clear specifications at every phase of production. Process validation confirms capability while providing confidence in product consistency.
Products: Quality Throughout GMP Lifecycle
All products must undergo constant testing, comparison, and quality assurance before being distributed to consumers. ISO 9001 identification traceability systems ensure complete product history from raw materials through distribution.
Procedures: Documented GMP Systems
GMP compliance heavily relies on personnel implementing documented procedures, with respective managers clear on job descriptions for each worker to avoid misunderstandings and reduce risks like overlapping responsibilities. Well-documented procedures provide consistency while supporting training and knowledge transfer.
Creating Effective GMP Compliance Posters for Your Facility
Effective GMP compliance posters combine visual appeal with practical information that personnel can quickly reference during operations. According to FDA guidance, CGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities.
Key design elements include clear hierarchies emphasizing the most critical information, color coding that highlights different requirement categories, and graphics that reinforce key concepts without cluttering displays. Text should be concise yet comprehensive, providing sufficient detail for understanding without overwhelming viewers.
Content organization should follow logical patterns that mirror workflow sequences, helping personnel locate relevant information quickly during operations. Poster good manufacturing practices displays should be updated regularly to reflect procedural changes and maintain relevance.
Strategic placement positions posters where personnel naturally look during operations, near equipment, at workstations, and in break areas, where they reinforce concepts during downtime. Multiple poster locations ensure visibility throughout facilities while addressing the specific needs of different operational areas.
Manufacturing facilities should develop poster rotation schedules that introduce new concepts regularly while maintaining displays of foundational principles. This balance prevents "poster blindness," where familiar displays become invisible through constant exposure.
GMP Implementation Strategies for Different Manufacturing Sectors
Pharmaceutical Manufacturing GMP Requirements
Current Good Manufacturing Practice regulations enforced by the FDA provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities, with adherence assuring the identity, strength, quality, and purity of drug products. Pharmaceutical manufacturers face stringent regulatory oversight requiring comprehensive quality systems.
GMP ISO 13485 differences comparisons help manufacturers understand overlapping requirements when implementing multiple quality standards simultaneously.
Medical Device Manufacturing GMP Compliance
Medical device manufacturers must integrate GMP principles with specific device regulations addressing design controls, risk management, and post-market surveillance. ISO 13485 traceability medical device identification requirements complement GMP expectations.
Poster good manufacturing practices displays in medical device facilities should emphasize device-specific requirements while reinforcing foundational GMP principles applicable across manufacturing sectors.
Food and Beverage GMP Production Standards
Food and beverage manufacturers implement GMP principles addressing contamination prevention, allergen control, and sanitation that protect consumer safety. Industry-specific regulations supplement general GMP requirements with detailed food safety protocols.
Cosmetic Manufacturing GMP Requirements
Cosmetic manufacturers face unique GMP requirements addressing ingredient safety, labeling accuracy, and contamination prevention specific to personal care products. While regulatory oversight may differ from pharmaceutical requirements, fundamental GMP principles remain applicable.
Common GMP Compliance Challenges and Practical Solutions
Manufacturing facilities implementing GMP principles face common challenges, including resource constraints, procedural complexity, personnel turnover, and resistance to change. Understanding these challenges enables proactive mitigation that strengthens compliance programs.
Resource limitations often constrain training budgets, technology investments, and quality staffing levels. Organizations can address these constraints through phased implementation approaches, leveraging technology for efficiency, and demonstrating return on investment that justifies additional resources.
Procedural complexity overwhelms personnel when documentation becomes excessive or disconnected from actual work practices. Simplification initiatives should streamline procedures while maintaining regulatory compliance, using visual aids and poster good manufacturing practices displays to communicate essential information effectively.
Personnel turnover disrupts quality culture while creating knowledge gaps that compromise operations. Comprehensive training programs, clear documentation, and systematic knowledge transfer processes mitigate turnover impacts while maintaining operational continuity.
How BPRHub Helps with GMP Compliance Management
BPRHub revolutionizes good manufacturing practices implementation through comprehensive Quality, Compliance, and Governance platforms that centralize all regulatory requirements within unified, intelligent systems. The platform transforms complex GMP compliance from an overwhelming administrative burden into a streamlined competitive advantage.
With automated document management systems, real-time compliance monitoring, and integrated training management, BPRHub ensures your manufacturing operations maintain continuous audit readiness while accelerating time-to-market. The platform's Unified Compliance Framework manages over 30 regulatory standards simultaneously, eliminating duplicate workflows that traditionally consume valuable resources.
BPRHub's intelligent automation captures quality data in real-time, generates regulatory reports automatically, and provides predictive analytics that identify potential compliance issues before they impact operations. This proactive approach reduces regulatory risk while freeing your team to focus on innovation and growth.
For manufacturers seeking expert guidance on GMP implementation, BPRHub's consulting services provide specialized support that accelerates compliance while building sustainable quality systems.
Stay audit-ready, every day. Streamline Good Manufacturing Practices with BPRHub.
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ Poster good manufacturing practices displays serve as constant visual reminders that reinforce training, communicate key principles, and demonstrate quality commitment during regulatory inspections
→ The 10 GMP principles cover all aspects of production from materials and premises to equipment, training, and personal hygiene, forming comprehensive frameworks for manufacturing excellence
→ The 5 Ps of GMP, People, Premises, Processes, Products, and Procedures, provide practical frameworks for understanding and implementing GMP requirements across manufacturing operations
→ FDA CGMP regulations provide systems assuring proper design, monitoring, and control of manufacturing processes that guarantee product identity, strength, quality, and purity
→ Effective GMP compliance poster displays a combination of visual appeal with practical information, strategic placement, and regular updates that maintain relevance and prevent poster blindness
→ BPRHub's comprehensive platform complements visual compliance tools through digital systems that centralize documentation, automate workflows, and provide real-time visibility into compliance status
Frequently Asked Questions
What are the 10 principles of Good Manufacturing Practices?
The 10 principles of GMP include: written procedures and instructions, following documented procedures, documentation of all activities, validation and change control, facilities and equipment design and maintenance, materials management, personnel training and competence, personal hygiene practices, quality control integration throughout production phases, and regular auditing through self-inspection and quality audits. These principles form the foundation for quality management systems, ensuring consistent product quality.
What is the difference between GMP and cGMP?
Good Manufacturing Practices (GMP) and current Good Manufacturing Practices (cGMP) are interchangeable terms, with GMP representing the basic regulations and cGMP emphasizing the requirement to use current, up-to-date technologies and systems. The "C" in cGMP stands for "current," requiring companies to use technologies and systems that are up-to-date to comply with regulations, as systems that were adequate 10-20 years ago may be less than adequate by today's standards.
What are the 5 main components of Good Manufacturing Practice?
The five main components of GMP, commonly referred to as the 5 Ps, are: People (all employees trained and adhering to processes), Premises (facilities properly designed and maintained), Processes (validated and controlled operations), Products (consistent testing and quality assurance), and Procedures (documented systems followed consistently). These components work together to ensure comprehensive quality management throughout manufacturing operations and align with ISO 9001 quality management principles.
Why are GMP compliance posters important in manufacturing facilities?
GMP compliance posters serve multiple critical functions in manufacturing environments. They provide constant visual reminders of key principles and requirements, reinforce training concepts that personnel receive during formal sessions, support regulatory compliance by demonstrating organizational commitment to quality during inspections, and help prevent quality issues by keeping critical information visible throughout operations. Effective good manufacturing practices complement comprehensive quality management systems, supporting a continuous improvement culture that reduces deviations and non-conformances.
What industries must follow Good Manufacturing Practices?
GMP applies to manufactured products such as food, cosmetics, and pharmaceutical goods. GMP guidelines are legislated by manufacturers' respective national governments and apply to food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. While specific requirements vary by industry and jurisdiction, fundamental GMP principles remain consistent across sectors, emphasizing contamination prevention, process control, and quality assurance through comprehensive compliance frameworks.
How often should GMP compliance posters be updated?
Good manufacturing practices displays should be reviewed and updated whenever procedures change, regulatory requirements are revised, audit findings identify knowledge gaps, or organizational assessments indicate that poster content has become outdated. Many manufacturing facilities implement quarterly poster rotation schedules that introduce new concepts while maintaining displays of foundational principles. The key is balancing consistency that allows personnel to internalize information with freshness that prevents poster blindness, where familiar displays become invisible through constant exposure, supported by digital documentation systems.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)
%20Poster%20Complete%20Implementation%20Guide.jpg)




%20(1).svg)

.avif)

