In today's competitive manufacturing landscape, good manufacturing practices represent the cornerstone of operational excellence and regulatory compliance. Manufacturing leaders who master GMP guidelines transform compliance from a cost center into a strategic advantage that drives sustainable growth and market expansion.
This comprehensive guide explores everything you need to know about good manufacturing practices examples, implementation strategies, and how modern compliance management, like BPRHub, empowers manufacturers to achieve audit-ready operations while scaling efficiently.
What is GMP, and why does it matter?
Good Manufacturing Practice (GMP) encompasses a comprehensive system of manufacturing standards designed to ensure consistent production of safe, effective, and high-quality products. These manufacturing regulations establish minimum requirements for the methods, facilities, and controls used in manufacturing, processing, packing, or holding products.
GMP standards serve as the foundation for quality assurance across multiple industries, creating a framework that protects consumers while enabling manufacturers to demonstrate their commitment to product safety and regulatory compliance.
What are the Core Purpose and Objectives?
Good manufacturing practices achieve three fundamental objectives
- Product Safety: Ensure products meet safety requirements and remain free from contamination
- Quality Consistency: Maintain uniform product quality across all production runs
- Regulatory Compliance: Meet legal requirements and prepare for regulatory inspections
These objectives align perfectly with manufacturers' business goals: protecting brand reputation, reducing liability risks, and accessing regulated markets with confidence.
Industries That Require GMP Compliance
Good manufacturing practices apply across numerous industries, each with specific regulatory requirements
- Pharmaceutical Manufacturing: FDA 21 CFR Parts 210 and 211
- Food and Beverage Production: FDA 21 CFR Part 117
- Medical Device Manufacturing: FDA 21 CFR Part 820
- Cosmetics Industry: FDA 21 CFR Part 700
- Dietary Supplement Manufacturing: FDA 21 CFR Part 111
Food manufacturers implement good manufacturing practices in the food industry through the FDA's Current Good Manufacturing Practices (CGMPs) regulations. Cosmetics companies follow 21 CFR Part 211 requirements, while supplement manufacturers comply with dietary supplement-specific GMP standards.
Take Control of GMP Compliance Before Your Next Audit- Speak with a GMP specialist today.
📍 Book a Demo
📧 hello@bprhub.com
Understanding Good Manufacturing Practice Guide
Definition and Scope of GMP Guidelines
A good manufacturing practice guide provides systematic instructions for implementing quality management systems that ensure consistent product quality and regulatory compliance. These guides translate complex regulatory requirements into actionable operational procedures.
Modern GMP guidelines address five critical areas: personnel training, facility design, equipment maintenance, process control, and documentation management. Each area requires specific standards and procedures tailored to individual manufacturing operations.
Key Regulatory Bodies (FDA, WHO, EMA)
Multiple regulatory agencies oversee good manufacturing practices globally:
FDA (Food and Drug Administration): Establishes GMP standards for US manufacturers and imports
WHO (World Health Organization): Develops international GMP standards for global harmonization
EMA (European Medicines Agency): Oversees GMP compliance within European markets
ICH (International Conference on Harmonisation): Coordinates global pharmaceutical standards
These agencies collaborate to harmonize good manufacturing regulations across international markets, enabling manufacturers to achieve global compliance through standardized quality systems.
International Standards and Harmonization
Good manufacturing practices continue evolving toward international harmonization. The ICH Q7 guidelines for Active Pharmaceutical Ingredients exemplify this trend, establishing unified standards that manufacturers can implement across multiple regulatory jurisdictions.
This harmonization reduces compliance complexity while maintaining rigorous safety and quality requirements. Manufacturers who adopt internationally harmonized good manufacturing practices gain competitive advantages in global markets.
What is the difference between GMP and cGMP (Current Good Manufacturing Practices)
Current Good Manufacturing Practices (cGMP) represent the modern evolution of traditional GMP standards. The "current" designation emphasizes that manufacturers must employ up-to-date technologies, equipment, and procedures that reflect current industry best practices.
cGMP standards require continuous improvement and adaptation to technological advances. Manufacturers cannot rely on outdated systems that may have been acceptable decades ago but fail to meet current safety and quality expectations.
Read More on Understanding Differences and Comparisons: GMP and ISO 13485 Standards
What are the 5 Main Components of GMP (The 5 P's)
People: Personnel Requirements and Training
Good manufacturing practices begin with properly trained personnel who understand their roles and responsibilities. Every employee must receive comprehensive training on GMP standards, safety procedures, and quality requirements specific to their job functions.
Effective personnel management includes:
- Initial GMP training for all new employees
- Regular refresher training to maintain competency
- Performance assessments to verify understanding
- Documentation of all training activities
Products: Quality Assurance and Testing Standards
Product quality assurance requires systematic testing and validation procedures that verify every product meets established specifications. Good manufacturing practices mandate comprehensive testing protocols for raw materials, in-process products, and finished goods.
Quality assurance systems must include:
- Incoming material inspection and testing
- In-process monitoring and control
- Finished product testing and release procedures
- Stability testing and shelf-life validation
Processes: Standardized Operating Procedures
Good manufacturing practices require documented procedures for every manufacturing process. These Standard Operating Procedures (SOPs) ensure consistent execution regardless of which personnel perform the work.
Process standardization includes:
- Detailed work instructions for each operation
- Equipment operating procedures
- Cleaning and maintenance procedures
- Change control procedures for process modifications
Procedures: Documentation and Compliance Guidelines
Documentation represents the backbone of good manufacturing practices. Every procedure, test result, and deviation must be properly documented and maintained according to regulatory requirements.
Documentation requirements include:
- Batch production records
- Laboratory test results
- Equipment maintenance logs
- Training records and certifications
Premises: Facility Design and Maintenance Requirements
Manufacturing facilities must meet specific design and maintenance standards that prevent contamination and ensure product quality. Good manufacturing practices establish requirements for facility layout, environmental controls, and maintenance procedures.
Facility requirements include
- Appropriate facility design and construction
- Environmental monitoring and control systems
- Cleaning and sanitation procedures
- Pest control and waste management systems
What are the 10 Fundamental Principles of GMP?
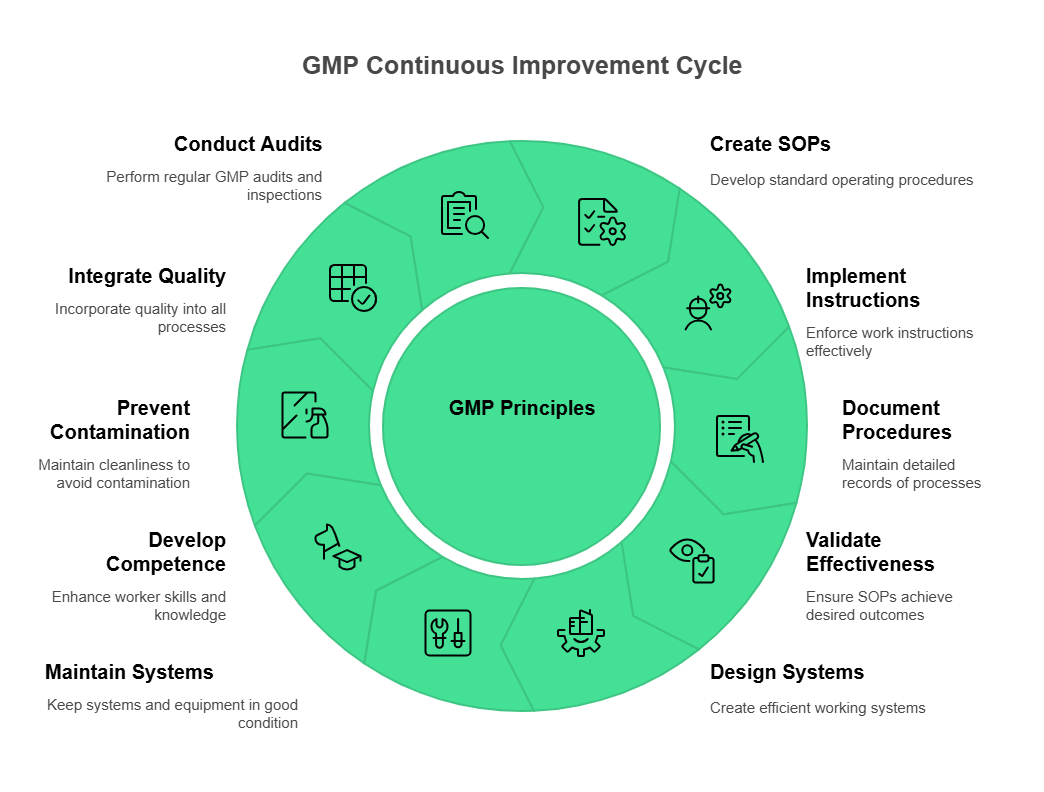
Creating Standard Operating Procedures (SOPs)
Good manufacturing practices require comprehensive SOPs that document every aspect of manufacturing operations. These procedures must be clear, detailed, and regularly updated to reflect current practices and regulatory requirements.
Effective SOPs include step-by-step instructions, safety precautions, quality control measures, and documentation requirements. They serve as the foundation for consistent operations and regulatory compliance.
Implementing and Enforcing Work Instructions
Work instructions translate SOPs into specific, detailed directions for individual tasks. Good manufacturing practices require that all personnel follow approved work instructions consistently.
Implementation includes regular training, performance monitoring, and corrective action procedures for deviations from established instructions.
Documentation of Procedures and Processes
Good manufacturing practices mandate comprehensive documentation of all procedures, processes, and results. This documentation demonstrates compliance and provides the evidence needed for regulatory inspections.
Documentation must be contemporaneous, accurate, and complete. Electronic documentation systems like BPRHub's platform ensure records remain accessible, searchable, and audit-ready.
Validation of SOP Effectiveness
Good manufacturing practices require ongoing validation that SOPs achieve their intended results. This validation includes process qualification, equipment validation, and cleaning validation.
Validation demonstrates that procedures consistently produce expected results under normal operating conditions.
Design and Use of Working Systems
Manufacturing systems must be designed to prevent contamination, mix-ups, and errors. Good manufacturing practices establish requirements for system design, implementation, and maintenance.
System design includes workflow patterns, material handling procedures, and quality control checkpoints that ensure product integrity throughout manufacturing.
Maintenance of Systems, Facilities, and Equipment
Good manufacturing practices require systematic maintenance of all systems, facilities, and equipment. Preventive maintenance programs ensure equipment operates within specified parameters and meets quality requirements.
Maintenance programs include scheduled inspections, calibration procedures, and repair documentation that demonstrates ongoing equipment reliability.
Development of Worker Job Competence
Personnel competence represents a critical component of good manufacturing practices. Workers must demonstrate the knowledge, skills, and abilities required for their assigned responsibilities.
Competence development includes initial training, ongoing education, and performance assessment procedures that ensure personnel maintain current capabilities.
Prevention of Contamination Through Cleanliness
Good manufacturing practices establish rigorous cleanliness standards that prevent contamination throughout manufacturing operations. These standards include facility cleaning, equipment sanitization, and personnel hygiene requirements.
Contamination prevention includes written cleaning procedures, validation of cleaning effectiveness, and ongoing monitoring to ensure continued compliance.
Quality Integration into Workflow
Quality management must be integrated into every aspect of manufacturing operations rather than treated as a separate function. Good manufacturing practices require that quality considerations guide all operational decisions.
Quality integration includes process controls, in-process testing, and quality oversight throughout production activities.
Regular GMP Audits and Inspections
Good manufacturing practices require regular internal audits and external inspections to verify ongoing compliance. These audits identify potential issues before they become regulatory violations.
Audit programs include scheduled internal audits, supplier audits, and preparation for regulatory inspections. BPR Hub streamlines audit management through automated scheduling, finding tracking, and corrective action management.
What are Good Manufacturing Practices?
Quality Management Systems
Good manufacturing practices examples in quality management systems demonstrate how manufacturers implement comprehensive quality controls. These systems integrate quality requirements into every aspect of operations.
Effective quality management systems include
- Quality planning and objective setting
- Process control and monitoring procedures
- Corrective and preventive action systems
- Management review and continuous improvement
Personnel Hygiene and Training Programs
Good manufacturing practices examples in personnel management show how manufacturers ensure workers maintain appropriate hygiene and competency levels. These programs protect product quality while supporting employee development.
Personnel programs include
- Hygiene training and monitoring procedures
- Competency assessment and certification
- Health monitoring and medical surveillance
- Performance evaluation and improvement plans
Facility Design and Environmental Controls
Good manufacturing practices examples in facility design demonstrate how manufacturers create environments that support product quality and safety. These designs incorporate workflow optimization and contamination prevention.
Facility design includes
- Logical workflow patterns that minimize contamination risks
- Environmental monitoring and control systems
- Appropriate lighting, ventilation, and temperature control
- Segregation of operations to prevent cross-contamination
Equipment Maintenance and Calibration
Good manufacturing practices examples in equipment management show how manufacturers ensure equipment operates within specified parameters. These programs prevent equipment-related quality issues.
Equipment programs include
- Preventive maintenance schedules and procedures
- Calibration programs for measuring equipment
- Equipment cleaning and sanitization procedures
- Change control for equipment modifications
Documentation and Record Keeping
Good manufacturing practices examples in documentation demonstrate how manufacturers maintain comprehensive records that support regulatory compliance. These systems ensure information remains accessible and accurate.
Documentation systems include
- Batch production records and device history records
- Laboratory testing results and certificates of analysis
- Training records and competency assessments
- Audit findings and corrective action records
Raw Material Control and Storage
Good manufacturing practices examples in material management show how manufacturers ensure incoming materials meet quality specifications. These controls prevent quality issues from entering production.
Material control systems include
- Incoming inspection and testing procedures
- Supplier qualification and monitoring programs
- Inventory management and traceability systems
- Storage condition monitoring and control
Production Process Controls
Good manufacturing practices examples in process control demonstrate how manufacturers maintain consistent production quality. These controls ensure products meet established specifications.
Process control systems include
- Process parameter monitoring and control
- In-process testing and verification procedures
- Batch record review and release procedures
- Process validation and revalidation programs
Quality Control and Testing Procedures
Good manufacturing practices examples in quality control show how manufacturers verify product quality through systematic testing. These procedures ensure products meet safety and efficacy requirements.
Quality control systems include
- Raw material testing and release procedures
- In-process testing and monitoring programs
- Finished product testing and certification
- Stability testing and shelf-life validation
What are the Good Manufacturing Practice Regulations?
FDA Regulations (21 CFR Parts 210, 211, 212)
Good manufacturing practice regulations under FDA jurisdiction establish comprehensive requirements for pharmaceutical, food, and medical device manufacturers. These regulations provide specific standards for facility design, personnel training, and quality control.
21 CFR Part 210 establishes general requirements for pharmaceutical cGMP, while Part 211 provides detailed implementation requirements. Part 212 addresses specific requirements for positron emission tomography drugs.
WHO GMP Guidelines for Pharmaceuticals
The World Health Organization's good manufacturing practice regulations provide international standards for pharmaceutical manufacturing. These guidelines support global harmonization while maintaining rigorous safety and quality requirements.
WHO GMP guidelines address
- Quality management systems
- Personnel training and competency
- Facility design and maintenance
- Equipment qualification and validation
European Union GMP Requirements (EudraLex Volume 4)
European Union good manufacturing practice regulations establish comprehensive requirements for pharmaceutical manufacturers operating within European markets. These regulations align with international standards while addressing specific European requirements.
EudraLex Volume 4 provides detailed guidance on
- Quality management systems
- Personnel qualifications and training
- Facility and equipment requirements
- Documentation and record keeping
ICH Q7 Guidelines for Active Pharmaceutical Ingredients
International Conference on Harmonisation (ICH) Q7 guidelines establish good manufacturing practice regulations for active pharmaceutical ingredient (API) manufacturing. These guidelines provide internationally harmonized standards that reduce regulatory complexity.
ICH Q7 addresses
- Quality management systems
- Personnel training and competency
- Facility design and maintenance
- Equipment qualification and validation
What are the Regulatory Compliance Requirements?
Documentation and Record Keeping Standards
Documentation standards establish requirements for record creation, maintenance, and retention that demonstrate compliance with regulatory requirements. Good manufacturing practice regulations mandate comprehensive documentation of all manufacturing activities.
Documentation systems must ensure accuracy, completeness, and accessibility while maintaining data integrity. Organizations implement electronic document management systems that streamline documentation processes while ensuring regulatory compliance.
Personnel Qualifications and Training
Personnel requirements establish standards for employee qualifications, training, and ongoing competency maintenance. Good manufacturing practice regulations require systematic approaches to personnel management that ensure appropriate knowledge and skills.
Training programs must address regulatory requirements, quality standards, and specific job functions. Organizations implement training management systems that track competency while ensuring maintained qualification levels.
Facility and Equipment Validation
Validation requirements establish standards for facility design, equipment installation, and operational qualification. Good manufacturing practice regulations require systematic validation that demonstrates suitable manufacturing conditions.
Validation activities include installation qualification, operational qualification, and performance qualification. Organizations implement validation management systems that ensure compliance with validation requirements while maintaining operational efficiency.
Quality Control and Testing Requirements
Quality control requirements establish standards for testing, analysis, and release procedures that ensure product quality. Good manufacturing practice regulations require comprehensive quality control programs that verify compliance with product specifications.
Testing programs must include appropriate methods, equipment, and documentation. Organizations implement laboratory information management systems that streamline testing while maintaining data integrity.
Inspection and Audit Procedures
Inspection requirements establish standards for regulatory inspections, internal audits, and supplier assessments. Good manufacturing practice regulations require systematic audit programs that verify compliance with quality standards.
Audit activities include system reviews, process observations, and documentation examinations. Organizations implement audit management systems that track findings while ensuring continuous improvement.
What are the Industry-Specific Regulations?
Pharmaceutical Manufacturing Requirements
Pharmaceutical regulations establish comprehensive requirements for drug product manufacturing that ensure patient safety and product efficacy. Good manufacturing practice regulations require systematic approaches to quality management that address all aspects of pharmaceutical production.
Pharmaceutical requirements include facility design, personnel qualification, equipment validation, and quality control systems. Organizations implement pharmaceutical quality systems that ensure regulatory compliance while maintaining operational efficiency.
Food and Beverage Industry Standards
Food industry regulations establish requirements for food manufacturing that ensure food safety and product quality. Good manufacturing practice regulations require systematic approaches to hazard analysis, preventive controls, and verification activities.
Food industry standards include facility design, personnel hygiene, equipment maintenance, and environmental controls. Organizations implement food safety management systems that ensure regulatory compliance while maintaining operational efficiency.
Medical Device Manufacturing Guidelines
Medical device regulations establish requirements for device manufacturing that ensure product safety and effectiveness. Good manufacturing practice regulations require systematic approaches to quality management that address device-specific requirements.
Medical device guidelines include design controls, risk management, and quality assurance systems. Organizations implement medical device quality systems that ensure regulatory compliance while maintaining product quality.
Cosmetics Industry Regulations
Cosmetics regulations establish requirements for cosmetic product manufacturing that ensure consumer safety and product quality. Good manufacturing practice regulations require systematic approaches to quality management that address cosmetic-specific requirements.
Cosmetics requirements include facility design, personnel training, and quality control systems. Organizations implement quality systems that ensure regulatory compliance while maintaining product quality.
What are the Implementation and Compliance Strategies?
Developing GMP Programs
Successful good manufacturing practices implementation requires systematic program development that addresses all regulatory requirements while supporting business objectives. This development process includes gap analysis, procedure development, and training implementation.
Program development includes:
- Current state assessment and gap analysis
- Procedure development and documentation
- Training program design and implementation
- Performance monitoring and improvement
Employee Training and Education
Good manufacturing practices depend on well-trained personnel who understand their roles and responsibilities. Training programs must address regulatory requirements, operational procedures, and quality standards.
Training programs include
- Initial GMP training for all personnel
- Job-specific training for assigned responsibilities
- Regular refresher training and updates
- Competency assessment and certification
Internal Audit Systems
Internal audits provide ongoing verification that good manufacturing practices remain effective and compliant. These audits identify potential issues before they become regulatory violations.
Audit systems include
- Scheduled audit programs and procedures
- Risk-based audit planning and execution
- Finding investigation and corrective action
- Continuous improvement implementation
Continuous Improvement Processes
Good manufacturing practices require ongoing improvement to maintain effectiveness and address changing regulatory requirements. Continuous improvement processes ensure GMP programs remain current and effective.
Improvement processes include
- Performance monitoring and measurement
- Trend analysis and risk assessment
- Corrective and preventive action implementation
- Management review and system updates
Technology and Digital Solutions
Modern good manufacturing practices benefit from digital solutions that automate documentation, streamline workflows, and provide real-time visibility into compliance status. These solutions reduce manual effort while improving accuracy and completeness.
Digital solutions include
- Electronic document management systems
- Automated workflow and approval processes
- Real-time dashboards and reporting
- Integration with existing business systems
What are the Benefits of GMP Implementation?
Enhanced Product Quality and Safety
Good manufacturing practices implementation results in measurable improvements in product quality and safety performance. Systematic approaches to quality management reduce defects, prevent contamination, and ensure consistent product characteristics.
Quality improvements include reduced customer complaints, fewer product recalls, and improved regulatory compliance. Organizations experience enhanced brand reputation and increased customer confidence through consistent quality delivery.
Regulatory Compliance Assurance
GMP implementation ensures compliance with regulatory requirements while reducing inspection findings and enforcement actions. Systematic approaches to compliance management provide audit readiness and regulatory confidence.
Compliance benefits include reduced regulatory risk, improved inspection outcomes, and enhanced market access. Organizations achieve sustainable compliance through the systematic implementation of good manufacturing practices.
Brand Reputation and Consumer Trust
Good manufacturing practices implementation enhances brand reputation through consistent quality delivery and regulatory compliance. Consumer trust increases when organizations demonstrate commitment to quality and safety.
Reputation benefits include increased customer loyalty, improved market position, and enhanced competitive advantage. Organizations leverage a quality reputation to support business growth and market expansion.
Operational Efficiency Improvements
GMP implementation streamlines operations through systematic processes, reduces variability, and improves predictability. Good manufacturing practices eliminate waste, reduce rework, and optimize resource utilization.
Efficiency improvements include reduced costs, improved productivity, and enhanced profitability. Organizations achieve operational excellence through the systematic implementation of quality management systems.
Risk Management and Liability Reduction
Good manufacturing practices implementation reduces risks associated with product quality, regulatory compliance, and operational performance. Systematic risk management prevents issues while minimizing liability exposure.
Risk reduction benefits include lower insurance costs, reduced legal exposure, and improved financial performance. Organizations achieve sustainable risk management through the comprehensive implementation of quality systems.
What are the Common GMP Challenges and Solutions?
Documentation Management
Good manufacturing practices require extensive documentation that can become overwhelming without proper systems. Many manufacturers struggle with document control, version management, and accessibility.
Documentation challenges include
- Paper-based systems that are difficult to search and maintain
- Version control issues that create confusion and errors
- Accessibility problems that slow decision-making processes
- Regulatory inspection preparation that requires significant resources
Employee Training and Compliance
Personnel training represents one of the most challenging aspects of good manufacturing practices implementation. Organizations must ensure all employees understand their responsibilities while maintaining current competencies.
Training challenges include
- Comprehensive training programs that cover all regulatory requirements
- Ongoing training updates that reflect regulatory changes
- Competency assessment and certification procedures
- Training record maintenance and accessibility
Equipment Maintenance and Validation
Good manufacturing practices require systematic equipment maintenance and validation that ensures consistent performance. Many manufacturers struggle with preventive maintenance scheduling and validation documentation.
Equipment challenges include
- Preventive maintenance scheduling and execution
- Validation documentation and record keeping
- Calibration program management and tracking
- Equipment change control and impact assessment
Supply Chain Management
Good manufacturing practices extend beyond internal operations to include supplier qualification and monitoring. Supply chain management requires ongoing verification that suppliers meet quality requirements.
Supply chain challenges include
- Supplier qualification and assessment procedures
- Ongoing monitoring and performance evaluation
- Supply chain risk assessment and mitigation
- Supplier change control and impact assessment
Regulatory Updates and Changes
Good manufacturing practice regulations continue evolving as regulatory agencies update requirements and issue new guidance. Manufacturers must stay current with these changes and update their procedures accordingly.
Regulatory challenges include
- Monitoring regulatory changes and updates
- Assessing impact on existing procedures and systems
- Implementing required changes within specified timeframes
- Training personnel on new requirements and procedures
How BPRHub Helps with GMP Practices?
Traditional GMP implementation often involves fragmented systems, manual documentation, and reactive compliance approaches that create operational inefficiencies and audit anxiety. Manufacturers struggle with disconnected processes, inconsistent documentation, and limited visibility into compliance status across multiple regulatory standards.
BPRHub transforms GMP implementation through its Unified Compliance Framework that centralizes over 30 regulatory standards, including FDA, ISO, and WHO requirements on a single platform. The system digitizes document analysis, automates audit workflows, and provides real-time visibility into compliance status across all manufacturing operations.
Organizations using BPRHub experience streamlined audit preparation, reduced compliance costs, and enhanced operational efficiency. The platform's automated workflows eliminate manual processes, reduce documentation errors, and ensure maintained audit readiness while freeing up resources for strategic growth initiatives.
BPRHub's integrated approach to GMP management enables manufacturers to scale operations without scaling compliance complexity. The system provides centralized control over quality management systems, automated training tracking, and real-time performance monitoring that supports sustainable compliance across expanding operations.
Eliminate Compliance Bottlenecks—Automate Your GMP Today With BPR Hub
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ Good Manufacturing Practices transform compliance from reactive burden to proactive competitive advantage through systematic quality management approaches
→ The 5 P's framework (People, Products, Processes, Procedures, Premises) provides a comprehensive foundation for GMP implementation across all manufacturing industries
→ Good manufacturing practices in the food industry require specialized approaches to hazard analysis, preventive controls, and environmental monitoring that ensure food safety
→ Digital transformation and Industry 4.0 technologies revolutionize GMP implementation through real-time monitoring, predictive analytics, and automated quality control systems
→ Successful GMP programs integrate quality management throughout manufacturing operations while maintaining operational efficiency and regulatory compliance
→ BPRHub enables manufacturers to achieve systematic GMP compliance through centralized standards management, automated workflows, and real-time compliance visibility
FAQ
Q. What are GMP guidelines?
GMP guidelines are systematic instructions that translate regulatory requirements into actionable operational procedures for manufacturing organizations. These guidelines address personnel training, facility design, equipment maintenance, process control, and documentation management to ensure consistent product quality and regulatory compliance across the pharmaceutical, food, medical device, and cosmetics industries.
Q. What are the 5 rules of GMP?
The 5 fundamental rules of good manufacturing practices are the "5 P's":
- People (trained personnel)
- Products (quality assurance)
- Processes (standardized procedures)
- Procedures (documentation guidelines)
- Premises (facility requirements)
These rules ensure comprehensive quality management systems that prevent contamination, maintain consistency, and demonstrate regulatory compliance through systematic approaches to manufacturing excellence.
Q. What are the 10 golden rules of GMP?
The 10 golden rules of good manufacturing practices include: creating comprehensive SOPs, implementing work instructions, documenting all procedures, validating SOP effectiveness, designing working systems, maintaining facilities and equipment, developing worker competence, preventing contamination, integrating quality into workflows, and conducting regular audits. These rules provide systematic approaches to operational excellence and regulatory compliance
Q. Who certifies GMP?
GMP compliance is verified through regulatory inspections rather than third-party certification. The FDA conducts inspections for US facilities, while international organizations like the WHO and EMA provide guidelines that national authorities enforce. Some industries utilize third-party auditing services to verify compliance with specific GMP standards before regulatory inspections.
Q. Who gives GMP guidelines?
GMP guidelines are established by regulatory agencies, including the FDA (United States), WHO (international), EMA (European Union), and other national authorities. Industry organizations like ICH develop harmonized guidelines that facilitate international compliance. These organizations collaborate to create consistent standards while addressing regional regulatory requirements.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

