Copyright © 2025 BPR Hub. All rights reserved.
Compliance Management Platform for Medical Manufacturers
Our all-in-one solution handles FDA 21 CFR Part 820, ISO 13485:2016, MDSAP, GMP, and more—creating a single source of truth for all your quality and compliance needs.
%20(1).svg)
Prevent costly product recalls
%20(1).svg)
Access real-time insights across global regulations
%20(1).svg)
Maintain continuous audit readiness for FDA inspections
Book a free demo
The all-in-one platform for
Quality, Compliance, and
Governance (QCG).
.avif)
30+ standards. One platform.

ISO 9001
.svg)
ISO 45001:2018

ISO 14001:2015

ISO 13485

ISO 17025
Before
BPR Hub
FDA, GMP or ISO rejections causing delays in product launches
Non-compliance risks disrupting production cycles
Product recalls impacting patient trust and revenue
Losing market opportunities due to non-compliance

Get ahead and stay ahead with one solution
1
Your complex development cycles are making it harder to conform to regulations.
Your complex development cycles are making it harder to conform to regulations.
Cut QA Workload in Half with Automated, Centralized Documentation
Centralize management for all essential documents like Design History Files (DHF), Device Master Records (DMR), technical files, material safety data sheets, and quality certificates with BPR Hub. Our automated version control and approval workflows ensure all your documents meet FDA requirements for electronic records and electronic signatures.
.webp)
2
Inconsistent records make it difficult for you to track and identify problem batches.
Inconsistent records make it difficult for you to track and identify problem batches.
Never Miss a Compliance Update, Stay Audit-Ready 24/7
Get comprehensive support for all medical regulations, including ISO 13485, FDA, EU MDR, MDSAP and many more— in one platform.
Our Standard Management Hub ensures that all your relevant standards are regularly updated, helping you remain compliant through process changes and avoiding any penalties or warning letters.

3
You are always worried about your equipment breaking down.
You are always worried about your equipment breaking down.
End-to-End Batch Traceability & Device History Management
Centralize manufacturing records, test results, and supplier data, to enable instant traceability, simplified CAPA investigations, and robust regulatory compliance across ISO 13485:2015 standards
%20(1).avif)
4
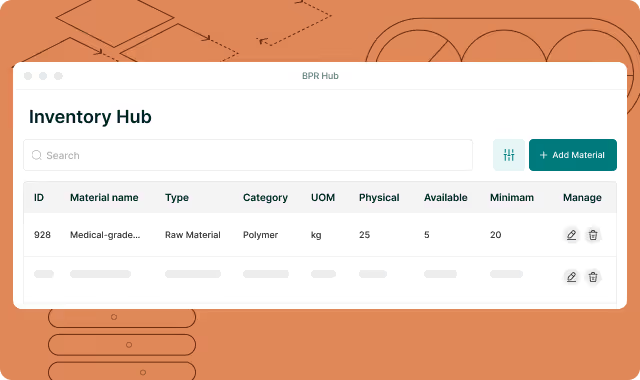
Poorly managed inventory and stock levels are hurting your profit margins.
Poorly managed inventory and stock levels are hurting your profit margins.
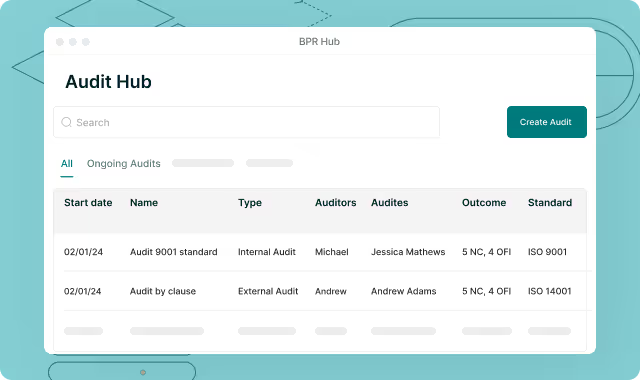
Pass FDA & Notified Body Audits with Confidence – Every Time
Streamline regulatory inspections with features for real-time compliance reporting, CAPA (Corrective and Preventive Action) tracking, and audit scheduling. Be prepared for FDA, Notified Body, or ISO certification audits at any time with comprehensive audit trail capabilities!
%20(1).avif)



Regulatory compliance for medical manufacturing can be complex. We’ve made it simple.
Schedule a demo
The all-in-one platform for
Quality, Compliance, and
Governance (QCG).
%20(1).webp)
.svg)
%20(1).svg)

.webp)
.webp)
.webp)
.webp)
.webp)
.webp)

%20(1).svg)

.avif)

