Manufacturing companies typically experience a cost of poor quality at 20% of total sales revenue, with Fortune Global 500 manufacturers collectively losing $864 billion annually to unplanned downtime, according to Senseye research. Systematic corrective and preventive action programs prevent these losses by transforming reactive problem-solving into proactive risk mitigation. CAPA represents the backbone of effective quality management, driving operational excellence and regulatory compliance.
This comprehensive guide explores how robust CAPA management systems eliminate recurring quality issues while building sustainable competitive advantages through systematic problem prevention and continuous improvement methodologies.
Understanding CAPA Corrective Action Fundamentals
CAPA corrective action forms the foundation of systematic quality improvement by addressing both immediate problems and their underlying causes. Unlike simple fixes that treat symptoms, effective corrective and preventive action programs eliminate root causes while preventing similar issues from recurring across operations.
The dual nature of CAPA addresses current non-conformances through corrective actions while implementing preventive measures that strengthen overall quality systems. This comprehensive approach transforms quality management from reactive firefighting into proactive operational excellence.
Core Definition of Corrective Actions in Quality Management
Corrective actions eliminate the causes of existing non-conformances or undesirable situations to prevent recurrence. These actions go beyond immediate containment to address systemic issues that could impact product quality, customer satisfaction, or regulatory compliance.
Effective corrective actions follow structured methodologies that identify root causes, implement sustainable solutions, and verify effectiveness through measurable outcomes. The process requires systematic investigation, comprehensive planning, and rigorous follow-up to ensure lasting improvement.
Quality management systems integrate corrective actions with broader improvement initiatives, creating synergies that amplify organizational learning while preventing isolated problem-solving approaches that miss systemic opportunities.
How Preventive Action Differs from Corrective Measures
Preventive action eliminates causes of potential non-conformances before they occur, representing a proactive approach to quality management. While corrective actions respond to existing problems, preventive actions anticipate and mitigate future risks based on trends, data analysis, and process understanding.
It's important to note that ISO 9001:2015 replaced the specific term "preventive action" with the broader concept of "risk-based thinking" in Clause 10.2, though the underlying preventive principles remain embedded throughout the standard. Other standards like ISO 13485 and IATF 16949 still maintain explicit preventive action requirements.
The distinction lies in timing and focus: corrective actions address known problems, while preventive actions prevent problems from developing. Both approaches share similar methodologies but differ in trigger mechanisms and validation approaches.
Successful organizations balance corrective and preventive activities, using data from corrective actions to identify opportunities for preventive measures while leveraging preventive actions to reduce the need for corrective interventions.
The Complete CAPA Process Breakdown
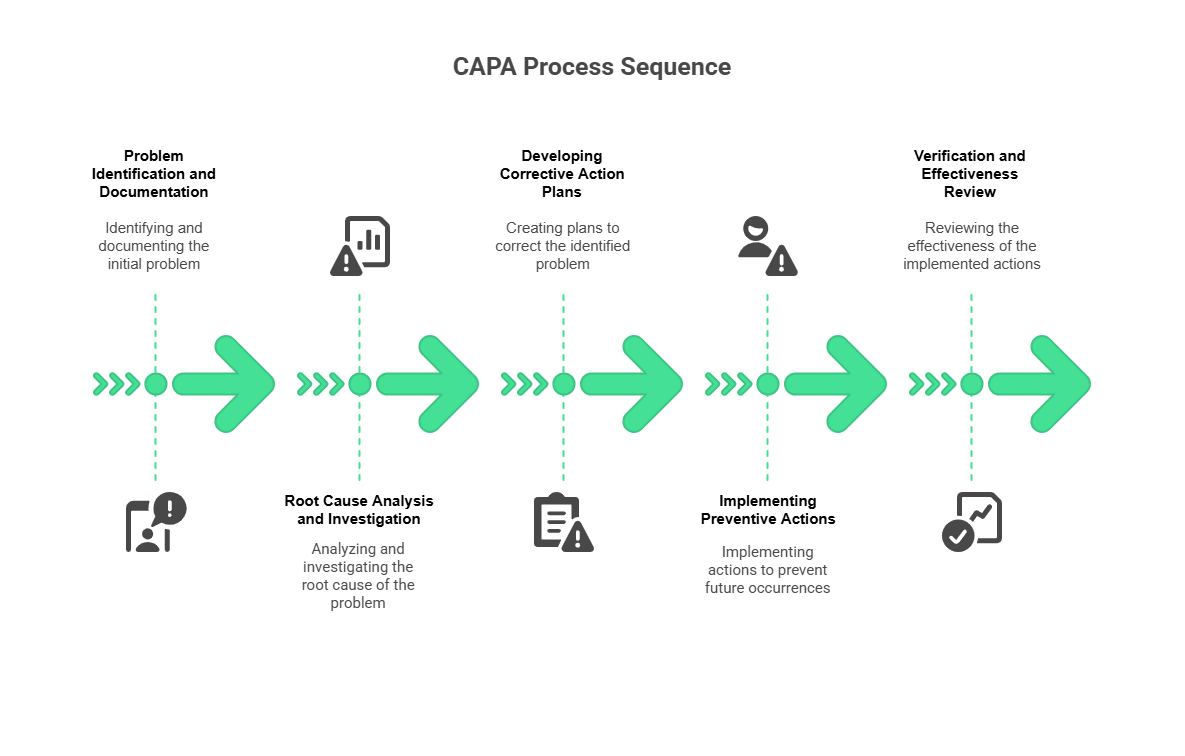
The CAPA process follows a systematic methodology that ensures thorough investigation, effective resolution, and sustainable improvement. This structured approach transforms ad hoc problem-solving into repeatable, measurable quality enhancement that supports continuous improvement objectives.
Each phase builds upon previous activities while maintaining documentation that supports regulatory compliance and organizational learning. The process emphasizes data-driven decision making and systematic verification of effectiveness.
Step 1 - Problem Identification and Documentation
Problem identification establishes the foundation for effective CAPA by clearly defining the issue, its scope, and potential impact on quality, safety, or compliance. Comprehensive documentation captures relevant details that support subsequent investigation and resolution activities.
Effective problem statements describe observable facts without assumptions about causes or solutions. The documentation includes timelines, affected products or processes, and preliminary risk assessments that guide resource allocation and priority determination.
Integration with quality management systems ensures that problem identification triggers appropriate escalation procedures while maintaining traceability between initial observations and final resolutions.
Step 2 - Root Cause Analysis and Investigation
CAPA investigation employs systematic methodologies to identify underlying causes rather than symptoms. Root cause analysis tools such as fishbone diagrams, 5-why analysis, and fault tree analysis provide structured approaches to understanding complex quality issues.
The investigation phase requires cross-functional collaboration to gather diverse perspectives and expertise. Teams examine processes, procedures, training, equipment, and environmental factors that could contribute to the identified problem.
Documentation throughout the investigation maintains objectivity while supporting conclusions with evidence. This rigor ensures that subsequent actions address actual causes rather than perceived issues.
Step 3 - Developing Corrective Action Plans
Corrective action planning translates investigation findings into specific, measurable actions that eliminate identified root causes. Effective plans include clear objectives, assigned responsibilities, target completion dates, and success criteria.
Action plans balance immediate containment needs with long-term systematic improvement. Short-term actions prevent problem recurrence while longer-term initiatives address systemic issues that could affect broader operations.
Resource allocation considerations ensure that corrective actions receive adequate support while integrating with existing improvement initiatives to maximize organizational impact.
Step 4 - Implementing Preventive Actions
Preventive actions implementation requires systematic change management that addresses processes, procedures, training, and monitoring systems. These changes often involve broader organizational impact than corrective actions, requiring careful planning and stakeholder engagement.
Implementation includes communication strategies that ensure affected personnel understand changes, receive appropriate training, and have the resources necessary for successful adoption. Change control processes maintain system integrity while enabling necessary improvements.
Monitoring during implementation identifies unexpected issues while verifying that preventive measures function as intended. This ongoing assessment enables mid-course corrections that enhance effectiveness.
Step 5 - Verification and Effectiveness Review
Verification activities confirm that implemented actions achieve intended objectives through measurable outcomes. Effectiveness reviews examine both immediate results and longer-term sustainability of improvements.
Key performance indicators track relevant metrics that demonstrate problem resolution and prevention effectiveness. These measurements provide objective evidence of CAPA success while identifying opportunities for further improvement.
Regular review cycles ensure that CAPA effectiveness remains current as processes, products, and operating conditions evolve. This ongoing assessment maintains improvement momentum while preventing degradation of achieved gains.
Streamline Your CAPA Process with BPRHub – Start Improving Today
📍 Book a Demo
📧 hello@bprhub.com
CAPA Requirements and Compliance Standards
CAPA requirements vary across industries but share common elements that emphasize systematic problem-solving, documentation, and effectiveness verification. Understanding these requirements enables organizations to design CAPA systems that satisfy multiple regulatory frameworks while supporting operational objectives.
Regulatory compliance represents a minimum standard that organizations should exceed through comprehensive CAPA programs that drive continuous improvement beyond basic compliance obligations.
FDA Corrective Action Guidelines and Expectations
FDA corrective action requirements under 21 CFR Part 820 Subpart J emphasize systematic approaches to quality improvement that protect patient safety while ensuring product quality. The FDA expects organizations to investigate quality issues thoroughly, implement effective corrective actions, and verify improvement sustainability.
According to FDA guidance documents, CAPA systems must address both immediate containment and long-term prevention through comprehensive root cause analysis and systematic implementation of improvements. The agency reviews CAPA effectiveness during inspections, expecting measurable evidence of problem resolution.
FDA expectations include timely investigation initiation, appropriate resource allocation, and regular effectiveness review. Organizations must demonstrate that CAPA programs contribute to overall quality system improvement rather than functioning as isolated problem-solving activities.
Medical Device CAPA Process Requirements
CAPA medical device requirements under FDA 21 CFR Part 820 mandate systematic approaches to quality improvement that address both corrective and preventive aspects. Medical device manufacturers must establish procedures for identifying quality problems, investigating root causes, and implementing effective solutions.
The regulation requires that CAPA procedures address product and process improvements while ensuring that changes don't negatively impact other aspects of quality systems. Documentation requirements include investigation records, action plans, implementation evidence, and effectiveness verification.
Medical device CAPA systems must integrate with design controls, risk management, and post-market surveillance activities to create comprehensive quality management approaches that support patient safety throughout product lifecycles.
Quality Management System Integration
CAPA quality integration with ISO 9001:2015 Clause 10.2 and other quality management standards creates synergies that amplify improvement effectiveness while reducing administrative burden. The current ISO 9001:2015 standard emphasizes corrective action within a risk-based thinking framework, integrating preventive concepts throughout the quality management system rather than as separate procedures.
Quality management system integration connects CAPA activities with management review, internal audit findings, and customer feedback to create holistic improvement programs. This integration ensures that improvement opportunities receive appropriate attention while preventing isolated problem-solving approaches.
The benefits of quality management systems extend beyond compliance to include operational efficiency improvements, cost reduction, and enhanced customer satisfaction that result from systematic CAPA implementation.
Why CAPA Management Matters for Business Success
CAPA management transforms quality challenges into competitive advantages by systematically eliminating problems while building organizational capability for continuous improvement. Effective CAPA programs reduce costs, improve customer satisfaction, and strengthen regulatory compliance posture.
Organizations with mature CAPA systems experience fewer quality crises, lower warranty costs, and improved operational efficiency compared to those relying on reactive problem-solving approaches.
Risk Reduction and Quality Improvement Benefits
CAPA programs significantly reduce quality risks by addressing root causes rather than symptoms. This systematic approach prevents problem recurrence while identifying systemic improvements that enhance overall operational performance.
Quality improvement through CAPA extends beyond immediate problem resolution to include process optimization, training enhancement, and system strengthening that creates sustainable competitive advantages.
Risk reduction benefits include lower product liability exposure, reduced regulatory inspection findings, and improved customer confidence that translates into market advantages and revenue growth opportunities.
Regulatory Compliance and Audit Readiness
Systematic CAPA implementation demonstrates organizational commitment to quality and compliance that regulatory agencies recognize during inspections. Well-documented CAPA programs provide evidence of proactive quality management that often results in favorable audit outcomes.
Audit readiness through CAPA includes comprehensive documentation, measurable effectiveness verification, and demonstrated continuous improvement that satisfies regulatory expectations while supporting business objectives.
Organizations with effective CAPA systems experience shorter audit durations, fewer inspection findings, and improved regulatory relationships that facilitate business growth and market expansion activities.
Cost Savings Through Problem Prevention
Problem prevention through systematic CAPA implementation delivers significant cost savings by eliminating waste, reducing rework, and preventing customer complaints. According to ASQ Cost of Quality methodology, organizations typically experience cost of poor quality at 20-25% of sales revenue, with effective CAPA programs substantially reducing these losses.
Cost savings include reduced scrap and rework, lower customer complaint handling costs, decreased warranty expenses, and improved operational efficiency that translates directly into improved profitability. Industry studies show that companies with mature CAPA systems experience a 15-30% reduction in quality-related costs within the first two years of implementation.
ISO 9001 quality management preventive maintenance principles align with CAPA approaches to create comprehensive cost reduction strategies that address both quality and operational efficiency objectives.

CAPA System Implementation Best Practices
CAPA system implementation requires systematic planning that addresses organizational needs, regulatory requirements, and operational constraints. Successful implementations balance comprehensiveness with practicality, ensuring that CAPA procedures add value while remaining manageable.
Effective implementations engage stakeholders throughout the organization, providing training and support that enables successful adoption while building internal expertise for long-term sustainability.
Building Effective CAPA Procedures
CAPA procedure development must address organizational needs while satisfying regulatory requirements. Effective procedures provide clear guidance for problem identification, investigation, action planning, implementation, and effectiveness verification.
Procedure development involves cross-functional teams that understand operational realities while bringing diverse perspectives to CAPA system design. This collaboration ensures that procedures are practical, comprehensive, and aligned with organizational culture.
ISO 9001 good documentation control principles apply to CAPA procedure development, ensuring that documentation supports effective implementation while remaining current and accessible.
Documentation Requirements and Standards
Documentation requirements for CAPA systems must balance comprehensiveness with usability. Effective documentation captures essential information while remaining accessible to users with varying technical backgrounds and experience levels.
Standards for CAPA documentation include clear problem statements, thorough investigation records, specific action plans, implementation evidence, and effectiveness verification results. Each element contributes to overall CAPA effectiveness while supporting regulatory compliance.
Documentation systems should integrate with existing quality management systems to eliminate duplicate record-keeping while ensuring traceability between CAPA activities and broader quality improvement initiatives.
Team Roles and Responsibilities
A clear role definition ensures that CAPA activities receive appropriate attention while preventing responsibility gaps that could compromise effectiveness. Successful CAPA systems define roles for problem identification, investigation leadership, action implementation, and effectiveness verification.
Responsibility assignments consider organizational structure, technical expertise, and resource availability while ensuring that CAPA activities don't overwhelm operational staff or compromise normal business activities.
Cross-functional teams bring diverse perspectives to CAPA activities while building organizational capability for systematic problem-solving and continuous improvement.
Common Implementation Challenges and Solutions
Implementation challenges often include resource constraints, competing priorities, and organizational resistance to systematic approaches. Successful implementations address these challenges through phased approaches, stakeholder engagement, and demonstrated value creation.
Solutions include starting with pilot programs that demonstrate CAPA value, providing comprehensive training that builds confidence, and integrating CAPA activities with existing business processes to minimize disruption.
Change management principles support CAPA implementation by addressing organizational concerns while building support for systematic improvement approaches that deliver measurable business benefits.
CAPA Management Software and Tools
CAPA management software transforms manual processes into automated workflows that improve efficiency while ensuring consistency and compliance. Modern software platforms provide comprehensive capabilities that support all aspects of CAPA implementation and management.
Software selection should consider organizational needs, regulatory requirements, integration capabilities, and user experience factors that affect adoption and long-term success.
Key Features of Corrective Action Software
Corrective action software must provide comprehensive capabilities that support problem identification, investigation management, action planning, implementation tracking, and effectiveness verification. Essential features include workflow automation, document management, and reporting capabilities.
Advanced features include integration with quality management systems, automated escalation procedures, statistical analysis capabilities, and dashboard reporting that provides real-time visibility into CAPA program performance.
User experience considerations ensure that software supports rather than impedes CAPA activities, encouraging adoption while maintaining data quality and process compliance.
Choosing the Right CAPA Management System
CAPA management system selection requires careful evaluation of organizational needs, technical requirements, and vendor capabilities. Successful selections balance functionality with usability while considering long-term scalability and integration requirements.
Evaluation criteria include regulatory compliance capabilities, integration options, user experience design, vendor support quality, and total cost of ownership considerations that affect long-term success.
Risk assessment example manufacturing guide principles apply to CAPA software selection, helping organizations identify and mitigate risks associated with system implementation and operation.
CAPA Training and Team Development
CAPA training builds organizational capability for systematic problem-solving while ensuring that personnel understand their roles in quality improvement initiatives. Effective training programs address both technical skills and quality system understanding.
Training approaches should consider diverse learning styles, varying experience levels, and specific organizational needs while building confidence in CAPA methodologies and tools.
Essential Skills for CAPA Teams
CAPA teams require diverse skills, including problem-solving methodologies, root cause analysis techniques, project management capabilities, and change management understanding. Technical skills must be balanced with communication and collaboration abilities.
Essential competencies include data analysis, process improvement, regulatory compliance understanding, and systematic thinking that enables comprehensive problem resolution and prevention activities.
ISO 9001 competence training requirements provide frameworks for developing CAPA team capabilities while ensuring that training investments deliver measurable performance improvements.
Building Internal CAPA Expertise
Internal expertise development reduces dependence on external consultants while building organizational capability for continuous improvement. Expertise development includes formal training, practical experience, and mentoring programs that transfer knowledge effectively.
Capability-building strategies include cross-functional project assignments, external training programs, professional development opportunities, and knowledge-sharing initiatives that distribute expertise throughout the organization.
Long-term expertise maintenance requires ongoing training, skill assessment, and capability updates that keep pace with evolving regulatory requirements and industry best practices.
Measuring CAPA Analysis Effectiveness
CAPA analysis effectiveness measurement provides objective evidence of program success while identifying opportunities for improvement. Effective measurement systems balance leading and lagging indicators that provide comprehensive performance visibility.
Measurement systems should align with organizational objectives while satisfying regulatory requirements for effectiveness verification and continuous improvement demonstration.
Key Performance Indicators for CAPA Programs
Key performance indicators for CAPA programs include problem resolution time, recurrence rates, customer satisfaction improvements, cost savings, and regulatory compliance metrics. These indicators provide comprehensive views of CAPA effectiveness.
Leading indicators, such as investigation timeliness and action plan completion rates, provide early warning of potential issues, while lagging indicators, such as problem recurrence and customer satisfaction, demonstrate long-term effectiveness.
Balanced scorecards integrate multiple perspectives on CAPA performance while ensuring that improvement efforts address all stakeholder needs and organizational objectives.
Continuous Improvement Through Data Analysis
Data analysis transforms CAPA information into actionable insights that drive continuous improvement. Analytical approaches include trend analysis, root cause frequency assessment, and effectiveness correlation studies that identify improvement opportunities.
Statistical analysis techniques help organizations identify patterns in quality issues, assess CAPA effectiveness, and predict future quality risks based on historical data and current trends.
The ISO 9001 management review template sample provides frameworks for incorporating CAPA data into management decision-making processes that drive organizational improvement.
Industry-Specific CAPA Applications
CAPA applications vary across industries based on regulatory requirements, risk profiles, and operational characteristics. Understanding industry-specific considerations enables organizations to tailor CAPA systems for maximum effectiveness.
Industry applications should balance regulatory compliance with operational efficiency while addressing unique risks and opportunities present in specific sectors.
Pharmaceutical and Life Sciences CAPA Compliance
CAPA compliance in the pharmaceutical and life sciences industries requires strict adherence to FDA regulations and international standards that emphasize patient safety and product quality. These industries face heightened scrutiny during regulatory inspections.
Pharmaceutical CAPA systems must address good manufacturing practice requirements, clinical trial data integrity, and post-market surveillance activities that span product lifecycles from development through commercialization.
GMP ISO 13485 differences comparisons help organizations understand regulatory variations while designing CAPA systems that satisfy multiple requirements efficiently.
Manufacturing Quality Control Integration
Manufacturing quality control integration requires CAPA systems that address production processes, supplier management, and customer requirements while maintaining operational efficiency and cost competitiveness.
Manufacturing CAPA applications include process improvement, equipment reliability enhancement, supplier corrective actions, and customer complaint resolution that address diverse quality challenges.
Integration with lean manufacturing principles creates synergies between CAPA activities and operational improvement initiatives that deliver enhanced value while reducing waste and inefficiency.
Creating Effective Corrective Action Reports
Corrective action reports provide comprehensive documentation of CAPA activities while communicating results to stakeholders, including management, regulatory agencies, and customers. Effective reports balance technical detail with accessibility.
Report quality directly impacts stakeholder confidence in CAPA effectiveness while supporting continuous improvement through knowledge sharing and lessons learned communication.
Report Structure and Content Requirements
The corrective action report structure should include problem description, investigation summary, root cause analysis, action plans, implementation evidence, and effectiveness verification results. Each section contributes to the overall report's comprehensiveness.
Content requirements vary based on audience needs, regulatory expectations, and organizational standards while maintaining consistency that supports comparative analysis and trend identification.
ISO 9001 quality record retention procedure principles apply to corrective action report management, ensuring that documentation remains accessible while meeting regulatory retention requirements.
Documentation Best Practices
Documentation best practices for corrective action reports include clear writing, logical organization, supporting evidence, and conclusions that flow from investigation findings. Visual aids such as flowcharts and graphs enhance understanding.
Best practices also include regular report reviews, template standardization, and continuous improvement of reporting processes that enhance communication effectiveness while reducing preparation time.
Quality documentation supports organizational learning while providing evidence of systematic improvement that satisfies regulatory requirements and stakeholder expectations.
How BPRHub Helps with CAPA Management
BPRHub's comprehensive Quality, Compliance, and Governance platform revolutionizes CAPA management by automating workflows while maintaining complete regulatory compliance. The platform transforms complex corrective and preventive action processes into streamlined, efficient systems that drive continuous improvement.
With integrated corrective action software capabilities, BPRHub enables organizations to identify problems quickly, investigate root causes systematically, and implement effective solutions while maintaining complete audit trails. The platform's intelligent automation ensures that CAPA activities follow proper procedures while accelerating resolution timeframes.
BPRHub's Unified Compliance Framework centralizes CAPA management alongside other quality system requirements, eliminating duplicate workflows while ensuring comprehensive coverage of regulatory obligations, including FDA 21 CFR Part 820, ISO 9001:2015, and ISO 13485 requirements. This integration reduces administrative burden while strengthening overall quality management effectiveness.
The platform's real-time reporting capabilities provide comprehensive visibility into CAPA program performance, enabling data-driven decisions that optimize resource allocation while maximizing improvement impact across manufacturing operations.
Boost Compliance & Cut Quality Costs – Power Your CAPA with BPRHub!
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ CAPA corrective action programs eliminate root causes of quality problems while preventing recurrence through systematic investigation and improvement methodologies
→ Preventive actions proactively address potential problems before they occur, creating competitive advantages through reduced quality risks and operational efficiency
→ CAPA management software automates workflows while ensuring regulatory compliance, accelerating problem resolution while maintaining comprehensive documentation
→ Effective CAPA systems integrate with quality management programs to create synergies that amplify improvement effectiveness while reducing administrative overhead
→ CAPA training builds organizational capability for systematic problem-solving, creating sustainable competitive advantages through enhanced quality management expertise
→ BPRHub's platform transforms complex CAPA processes into streamlined workflows that drive continuous improvement while ensuring complete regulatory compliance and audit readiness
FAQ
Q. What is CAPA, and why is it important for manufacturing companies?
CAPA (Corrective and Preventive Action) is a systematic approach to identifying, investigating, and resolving quality problems while preventing their recurrence. For manufacturing companies, CAPA is crucial because it transforms reactive problem-solving into proactive quality management that reduces costs, improves customer satisfaction, and ensures regulatory compliance. Effective CAPA programs eliminate recurring quality issues while building organizational capability for continuous improvement that creates sustainable competitive advantages.
Q. What are the main steps in the CAPA process?The CAPA process follows five main steps based on FDA 21 CFR Part 820 and ISO quality management standards: problem identification and documentation, root cause analysis and investigation, developing corrective action plans, implementing preventive actions, and verification and effectiveness review. Each step builds upon previous activities while maintaining comprehensive documentation that supports regulatory compliance. The systematic approach ensures that problems are thoroughly understood, effectively resolved, and prevented from recurring through sustainable improvements.
Q. How does CAPA differ from regular quality control activities?
CAPA differs from regular quality control by focusing on systematic root cause elimination rather than symptom treatment. While quality control activities detect and contain problems, CAPA investigates underlying causes and implements comprehensive solutions that prevent recurrence. Corrective and preventive action programs address both immediate issues and systemic improvements, creating long-term quality enhancement that extends beyond individual problem resolution to strengthen overall operational performance and regulatory compliance.
Q. What regulatory requirements apply to CAPA programs?
CAPA requirements vary by industry but commonly include FDA 21 CFR Part 820 for medical devices, ISO 9001:2015 Clause 10.2 for quality management systems, and industry-specific guidelines such as ISO 13485 for medical devices and IATF 16949 for automotive. These regulations require systematic approaches to problem identification, investigation, resolution, and effectiveness verification. Organizations must maintain comprehensive documentation that demonstrates CAPA effectiveness while ensuring that improvement activities address both corrective and preventive aspects of quality management.
Q. How can organizations measure the effectiveness of their CAPA programs?
CAPA analysis effectiveness is measured through key performance indicators, including problem resolution time, recurrence rates, cost savings, customer satisfaction improvements, and regulatory compliance metrics based on ASQ Cost of Quality principles. Leading indicators, such as investigation timeliness, provide early performance insights while lagging indicators, such as problem recurrence, demonstrate long-term effectiveness. Regular effectiveness reviews using statistical analysis and trend identification ensure that CAPA programs deliver measurable improvements while identifying opportunities for further enhancement.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

