Manufacturing organizations waste an average of 21% of their productive time searching for documents or recreating information that already exists, according to productivity research studies by McKinsey & Company. ISO 9001 document control transforms this inefficiency into a competitive advantage by establishing systematic approaches to managing documented information that accelerate operations while ensuring audit readiness.
Clause 7.5.3 control of documented information provides the framework for maintaining accurate, accessible, and secure documentation that supports quality objectives. This comprehensive guide breaks down implementation strategies that turn document management from an administrative burden into an operational excellence driver.
What is ISO 9001 Clause 7.5.3 Control of Documented Information?
ISO 9001 Clause 7.5.3 establishes requirements for controlling documented information throughout its lifecycle, from creation through disposal. This clause replaces the previous document control and record control requirements, creating a unified approach that addresses both external documents and internal quality system documentation.
The clause emphasizes that organizations must ensure documented information remains available, suitable for use, and adequately protected. This systematic approach prevents document-related quality failures while supporting continuous improvement initiatives.
Understanding Documented Information in Quality Management Systems
Quality management systems rely on documented information to ensure consistent processes, capture institutional knowledge, and demonstrate compliance with ISO 9001 standard requirements. This information includes procedures, work instructions, specifications, and records that support quality objectives.
Documented information serves multiple purposes: guiding personnel through complex processes, providing evidence of conformity, and supporting management decision-making. Effective control ensures that this information remains accurate, current, and accessible when needed.
The shift from "documents and records" to "documented information" reflects the modern reality of digital documentation while maintaining the same fundamental control principles that ensure quality system effectiveness.
Key Components of ISO Document Control
ISO document control encompasses identification, distribution, access, retrieval, use, storage, preservation, and disposition of documented information. Each component contributes to overall system effectiveness while preventing document-related quality issues.
Identification requirements ensure that documents can be uniquely recognized and retrieved when needed. Distribution controls guarantee that current versions reach appropriate personnel while preventing the use of obsolete information.
Access controls balance information availability with security requirements, ensuring that sensitive information remains protected while operational needs are met. These controls form the foundation of effective documentation control systems.
Differences from Previous ISO 9001 Documentation Requirements
ISO 9001 documentation requirements evolved significantly in the 2015 revision, moving from prescriptive document lists to risk-based approaches that allow organizations to determine appropriate documentation levels. This flexibility enables tailored solutions that match organizational complexity and risk profiles.
Previous versions mandated specific documents like quality manuals and documented procedures. The current ISO 9001 version 2015 approach focuses on ensuring that necessary documented information exists without prescribing specific formats or structures.
This evolution reflects modern document management practices while maintaining the fundamental control principles that ensure quality system effectiveness. Organizations can now optimize their documentation approaches while meeting regulatory requirements.
Streamline your ISO 9001 document control with BPRHub
📍 Book a Demo
📧 hello@bprhub.com
Essential ISO 9001 Documentation Requirements for Clause 7.5.3
ISO 9001 documentation requirements under Clause 7.5.3 focus on ensuring that documented information effectively supports quality system implementation. The standard requires organizations to maintain documented information necessary for quality system effectiveness and evidence of conformity.
Organizations must determine what documented information they need based on their context, complexity, and competence of personnel. This risk-based approach enables optimized documentation that supports operations without creating unnecessary administrative burden.
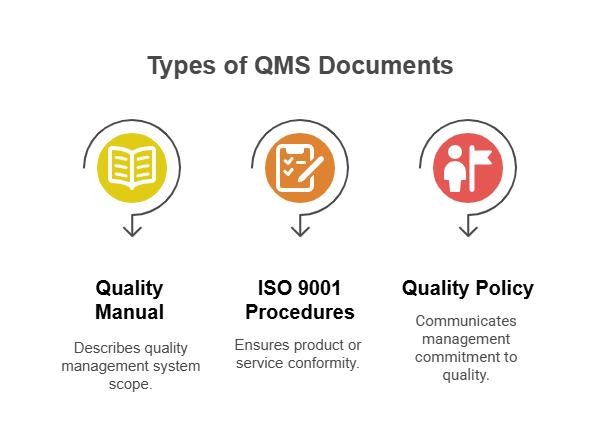
Types of QMS Documents Covered
QMS documents under Clause 7.5.3 include all forms of documented information that support quality system implementation. This encompasses policies, procedures, work instructions, specifications, plans, and records that demonstrate conformity and system effectiveness.
The scope includes both internally generated documents and external documents needed for quality system planning and operation. Integration of external documents like customer specifications and regulatory requirements ensures comprehensive coverage.
Quality Manual Documentation
Quality manual requirements have evolved significantly under ISO 9001 version 2015. While no longer mandatory, many organizations continue to maintain quality manuals as effective communication tools that describe their quality management system scope and approach.
Modern quality manuals serve as navigation tools that help personnel understand how quality system elements connect and support organizational objectives. They provide context for detailed procedures while demonstrating management commitment to quality.
When organizations choose to maintain quality manuals, they must ensure these documents remain current and accurately reflect actual quality system implementation.
ISO 9001 Procedures and Work Instructions
ISO 9001 procedures must be documented when their absence could affect product or service conformity. This determination depends on process complexity, personnel competence, and the potential impact of process variations on quality outcomes.
Work instructions provide detailed guidance for specific tasks, ensuring consistent execution across different operators and shifts. These documents bridge the gap between high-level procedures and actual work performance.
Effective procedures and work instructions balance sufficient detail with practical usability, ensuring that personnel can follow them efficiently while maintaining quality standards. ISO 9001 good documentation control practices enhance implementation effectiveness.
Quality Policy Documentation
Quality policy documentation communicates management commitment to quality and provides a framework for quality objectives. This foundational document must be available to relevant interested parties and maintained as documented information.
The quality policy should be appropriate to the organizational purpose and context, provide a framework for setting quality objectives, and include a commitment to satisfying applicable requirements and continual improvement.
Regular review and communication of the quality policy ensures that it remains relevant and effectively guides organizational quality efforts.
Mandatory vs Optional Documented Information
Documented information requirements fall into two categories: mandatory information specified by the ISO 9001 standard and optional information determined by organizations based on their specific needs and risk assessments.
Mandatory documented information includes quality policy, quality objectives, scope of quality management system, and specific procedures or information required by the standard. Organizations must maintain this information regardless of their size or complexity.
Optional documented information addresses organizational needs beyond minimum requirements. This might include additional procedures, work instructions, or guidance documents that support effective implementation based on organizational context and risk profile.
How to Implement QMS Document Control Systems
QMS document control implementation requires systematic planning that addresses document creation, approval, distribution, use, and maintenance throughout the document lifecycle. Effective systems balance control with accessibility, ensuring that personnel can easily access current information.
Successful implementation considers organizational structure, technology capabilities, and user needs to create practical systems that support rather than hinder operations. The goal is seamless document management that becomes invisible to users while providing robust control.
Setting Up Documentation Control Systems
Documentation control systems must address identification, storage, protection, retrieval, retention, and disposition of documented information. Modern systems typically leverage digital technologies to provide efficient, secure, and scalable solutions.
System architecture should consider current and future needs, including integration with other business systems and scalability requirements. Cloud-based solutions often provide cost-effective approaches for smaller organizations, while enterprise systems may better serve larger, complex operations.
Effective systems provide role-based access controls, automated workflow capabilities, and comprehensive audit trails that support both operational needs and compliance requirements.
Document Identification Methods
Document identification systems must uniquely identify each piece of documented information throughout its lifecycle. Effective identification schemes facilitate retrieval while providing information about document type, revision status, and applicable organizational units.
Common identification approaches include hierarchical numbering systems, alphanumeric codes, or database-driven unique identifiers. The chosen method should be intuitive for users while providing necessary control capabilities.
Automated identification systems reduce manual errors while ensuring consistency across all documented information. Integration with document management systems provides seamless identification throughout the document lifecycle.
Version Control Procedures
Version control procedures ensure that personnel always access current, approved documented information while maintaining historical versions for reference and compliance purposes. Effective version control prevents confusion and errors that could impact quality.
Automated version control systems provide time-stamped audit trails showing when changes occurred, who made them, and why they were necessary. This information supports both operational troubleshooting and compliance demonstrations.
Clear version identification helps users immediately recognize document currency while automated distribution ensures that updates reach appropriate personnel promptly.
Access Control Mechanisms
Access control mechanisms balance information security with operational needs, ensuring that personnel can access necessary documented information while protecting sensitive or confidential content. Role-based access controls provide flexible, scalable security.
Modern systems provide granular access controls that can be tailored to specific roles, departments, or projects. This flexibility ensures that personnel receive appropriate access while maintaining necessary security controls.
Automated access management reduces administrative overhead while ensuring that access rights remain current as personnel roles change or projects evolve.
ISO 9001 Document Management Best Practices
ISO 9001 document management best practices focus on creating user-friendly systems that encourage compliance while providing robust control capabilities. Effective systems make it easier to do things right than to do them wrong.
Best practices include establishing clear document standards, implementing automated workflows, and providing comprehensive training that ensures personnel understand both system capabilities and their responsibilities.
Regular system reviews and user feedback help identify improvement opportunities while ensuring that systems continue meeting organizational needs as they evolve.
Digital vs Physical Document Storage
Digital document storage offers significant advantages over physical storage, including improved accessibility, enhanced security, automated backup capabilities, and reduced storage costs. Modern organizations typically implement primarily digital systems with limited physical storage for specific requirements.
Digital systems provide full-text search capabilities, automated workflow routing, and comprehensive audit trails that greatly enhance document management effectiveness. Integration with other business systems provides seamless information flow.
Physical document storage may still be required for certain regulated industries or specific document types, but should be minimized where possible to realize digital benefits.
Document Approval Workflows
Document approval workflows ensure that documented information receives appropriate review and authorization before implementation. Automated workflows accelerate approval processes while maintaining necessary controls and audit trails.
Effective workflows route documents to appropriate reviewers based on content type, organizational impact, and established approval authorities. Parallel review capabilities can significantly reduce approval cycle times for complex documents.
Automated notifications and escalation procedures ensure that approvals don't become bottlenecks while providing management visibility into approval status and potential delays.
Distribution and Retrieval Systems
Distribution and retrieval systems ensure that current documented information reaches appropriate personnel while providing efficient access when needed. Modern systems automate distribution while providing comprehensive access analytics.
Automated distribution systems ensure that updates reach all affected personnel immediately upon approval, eliminating delays that could result in the use of obsolete information. Push notification capabilities alert users to critical updates.
Powerful search and retrieval capabilities help personnel quickly locate needed information, reducing time waste while improving operational efficiency. ISO 9001 quality record retention procedures support long-term access requirements.

ISO 9001 Compliance Requirements for Document Control
ISO 9001 compliance requires that organizations demonstrate systematic control over documented information throughout its lifecycle. Effective control supports both operational excellence and audit readiness by ensuring that documentation accurately reflects actual practices.
Compliance demonstrations focus on showing that documented information is available when needed, suitable for its intended use, and adequately protected from loss, misuse, or deterioration.
Meeting ISO 9001 Standard Requirements
ISO 9001 standard requirements for document control emphasize ensuring that documented information effectively supports quality system implementation. Organizations must demonstrate that their control systems address all lifecycle stages from creation through disposal.
Compliance requires evidence that documented information remains legible, readily identifiable, and retrievable throughout required retention periods. Protection measures must prevent deterioration, loss, or unauthorized modification.
Regular audit activities help verify that document control systems operate effectively while identifying opportunities for improvement. ISO 9001 questions and answers for audits provide preparation guidance.
Legibility and Readability Standards
Legibility and readability standards ensure that documented information can be effectively used by intended personnel. These standards address both physical and digital document formats to ensure consistent accessibility.
Standards should address font sizes, color contrasts, formatting conventions, and language requirements that support effective communication. Consideration of diverse user needs ensures inclusive access to necessary information.
Regular reviews help identify documents that may be difficult to read or understand, enabling proactive improvements before accessibility issues impact operations.
Protection and Security Measures
Protection and security measures safeguard documented information from unauthorized access, modification, or destruction while ensuring availability for authorized users. These measures must balance security with operational accessibility.
Physical protection addresses secure storage, environmental controls, and access restrictions that prevent document deterioration or loss. Digital protection includes backup systems, access controls, and cybersecurity measures.
Security measures should be proportionate to information sensitivity and criticality, ensuring appropriate protection without creating unnecessary barriers to legitimate access.
Retention and Disposal Procedures
Retention and disposal procedures ensure that documented information remains available throughout required periods while enabling secure destruction when retention periods expire. Clear procedures prevent premature disposal while avoiding excessive storage costs.
Retention periods should consider regulatory requirements, organizational needs, and potential future uses. Automated retention management systems help ensure compliance while reducing administrative overhead.
Secure disposal procedures protect sensitive information while providing audit trails that demonstrate appropriate handling of confidential or proprietary content.
ISO Requirements for Document Updates
ISO requirements for document updates ensure that documented information remains current and accurately reflects actual practices. Effective update processes balance change control with operational agility.
Update procedures must address change identification, impact assessment, approval requirements, and implementation coordination. These procedures ensure that changes are properly evaluated and implemented without disrupting operations.
Change history maintenance provides audit trails that support troubleshooting and compliance demonstrations while enabling rollback if updates create unexpected issues.
Change Control Processes
Change control processes ensure that modifications to documented information receive appropriate evaluation and approval before implementation. These processes prevent unauthorized changes while enabling necessary updates.
Effective change control includes impact assessment, stakeholder review, approval authorization, and implementation coordination. Risk-based approaches focus control efforts on changes with the highest potential impact.
Automated change control systems provide workflow management, approval tracking, and implementation coordination that streamlines change processes while maintaining necessary controls.
Obsolete Document Management
Obsolete document management prevents the use of superseded documented information while maintaining historical records for reference and compliance purposes. Clear identification and removal procedures prevent confusion and errors.
Automated systems can immediately flag obsolete documents while maintaining secure archives for future reference. This approach eliminates the risk of accidentally using outdated information.
Historical document retention supports troubleshooting, trend analysis, and compliance demonstrations while avoiding operational confusion about current requirements.
Review and Revision Cycles
Review and revision cycles ensure that documented information remains current and effective through regular evaluation and updates. These cycles balance stability with continuous improvement opportunities.
Scheduled reviews help identify documents that need updating while enabling proactive improvements before issues arise. Risk-based review frequencies focus attention on documents with the highest impact or change rates.
User feedback integration helps identify practical improvements that enhance document effectiveness while maintaining compliance with ISO 9001 standard requirements.
ISO 9001 Training for Document Control Implementation
ISO 9001 training for document control ensures that personnel understand their roles and responsibilities in maintaining effective documented information control. Comprehensive training covers both system operation and compliance requirements.
Training programs should address document creation, approval, distribution, use, and maintenance responsibilities specific to different organizational roles. Regular refresher training ensures continued competence as systems evolve.
Staff Training on ISO Documentation
ISO documentation training helps personnel understand how document control supports quality objectives while providing practical guidance for daily interactions with documented information. Training should be tailored to specific roles and responsibilities.
Effective training combines theoretical understanding with practical exercises that demonstrate proper document handling procedures. Hands-on experience with document management systems builds confidence and competence.
Regular training updates ensure that personnel remain current with system changes and evolving best practices. ISO 9001 competence training requirements provide additional guidance.
Competency Requirements for Document Controllers
Document controllers require specialized competency in document management principles, system operation, and regulatory requirements. These personnel serve as subject matter experts who support broader organizational document control efforts.
Competency requirements should address both technical skills and understanding of quality management principles. Regular competency assessment ensures that document controllers maintain the necessary knowledge and skills.
Professional development opportunities help document controllers stay current with evolving best practices and technological advances that can improve system effectiveness.
Training Records and Documentation
Training records provide evidence of personnel competency in document control procedures while supporting compliance demonstrations during audits. Comprehensive records include training content, completion dates, and competency assessments.
Automated training management systems can track completion, schedule refresher training, and generate compliance reports that support audit preparation. Integration with document management systems provides seamless record maintenance.
Regular review of training effectiveness helps identify improvement opportunities while ensuring that training programs continue meeting organizational needs.
Common Challenges in ISO Document Management
ISO document management implementation often encounters challenges related to technology integration, organizational change management, and process standardization. Understanding common challenges enables proactive mitigation strategies.
Successful implementations address these challenges through careful planning, stakeholder engagement, and phased rollout approaches that minimize disruption while building user confidence.
Technology Integration Issues
Technology integration challenges arise when document management systems must interface with existing business systems or when legacy technology creates compatibility issues. Careful planning and phased implementation help minimize these challenges.
Common integration issues include data migration, system compatibility, user training, and workflow redesign. Professional implementation support can help navigate complex technical requirements while ensuring successful outcomes.
Modern cloud-based systems often provide better integration capabilities while reducing on-premises infrastructure requirements. Platform selection should consider both current and future integration needs.
Multi-location Document Control
Multi-location operations create additional complexity for document control, particularly when different locations have varying technology capabilities or regulatory requirements. Centralized systems with local access capabilities often provide effective solutions.
Standardized procedures and technology platforms help ensure consistent document control across all locations while allowing for necessary local adaptations. Regular communication and training support implementation consistency.
Network connectivity and security considerations become more complex in multi-location environments, requiring careful planning to ensure reliable access while maintaining necessary protection.
External Document Management
External documents like customer specifications, regulatory requirements, and supplier documentation require special handling to ensure currency and accessibility. These documents often have different revision cycles and approval authorities than internal documents.
Effective external document management includes identification of required external documents, responsibility assignment for monitoring updates, and integration with internal document control systems.
Automated monitoring systems can track external document updates while providing alerts when new versions become available. This proactive approach helps prevent the use of obsolete external information.
ISO 9001 Clauses Related to Document Control
ISO 9001 clauses related to document control extend beyond Clause 7.5.3 to include requirements throughout the standard that depend on effective documented information management. Understanding these connections enables integrated implementation approaches.
Integration with other clauses ensures that document control supports broader quality management system effectiveness while avoiding duplicate or conflicting requirements.
Connection to Other ISO 9001 Clauses Explained
ISO 9001 clauses explain document control, showing how documented information supports requirements throughout the standard. For example, Clause 4.4 requires process documentation, while Clause 8.5 requires operational control documentation.
Management review requirements in Clause 9.3 depend on documented information about quality system performance. Corrective action requirements in Clause 10.2 require documentation of nonconformities and correction activities.
Understanding these connections helps organizations develop integrated documentation strategies that efficiently address multiple requirements without duplication or gaps.
Integration with ISO 9001 Quality Management System
ISO 9001 quality management system integration ensures that document control supports all quality system elements rather than operating as an isolated function. This integration creates synergies that enhance overall system effectiveness.
Document control integration touches every aspect of the quality system, from planning and resource management through operation and improvement activities. Effective integration makes document management invisible to users while providing robust control.
The benefits of quality management systems are enhanced when document control effectively supports system implementation and continuous improvement.
ISO 9001 Manual and Document Templates
ISO 9001 manual and document templates provide starting points for organizations developing their documentation frameworks. While quality manuals are no longer mandatory, many organizations find them valuable for communication and training purposes.
Templates should be adapted to the organizational context rather than used without modification. Generic templates rarely address specific organizational needs and may create unnecessary complexity.
Creating Document Control Procedures
Document control procedures establish systematic approaches to managing documented information throughout its lifecycle. These procedures should address organization-specific requirements while meeting ISO 9001 standard expectations.
Effective procedures balance comprehensive control with practical usability, ensuring that document management supports rather than hinders operations. User input during procedure development helps ensure practical applicability.
Procedures should be tested through pilot implementations before full deployment, enabling refinement based on actual user experience and operational requirements.
Document Format Standards
ISO 9001 PDF and other format standards ensure consistency and accessibility across all documented information. Standardized formats reduce training requirements while improving document's professional appearance.
Format standards should consider user needs, technology capabilities, and regulatory requirements. Accessible formats support diverse user needs while maintaining necessary control capabilities.
Version control considerations should influence format selection, ensuring that chosen formats support effective change management and historical preservation.
Template Development Guidelines
Template development guidelines ensure that document templates effectively support organizational needs while maintaining consistency with ISO 9001 standard requirements. Good templates accelerate document creation while ensuring compliance.
Templates should include required elements like identification, approval signatures, and revision tracking while providing flexibility for content adaptation. Overly rigid templates can hinder effective communication.
Regular template review and user feedback help identify improvement opportunities while ensuring that templates continue to meet evolving organizational needs.
ISO 9001 Certification and Document Control Audits
ISO 9001 certification audits place significant emphasis on document control effectiveness, as documented information provides evidence of quality system implementation and compliance. Audit preparation should focus on demonstrating system effectiveness rather than just compliance.
Auditors evaluate both document control systems and evidence of their effective implementation throughout the organization. This includes reviewing document content, currency, and evidence of actual use.
Audit Preparation for Document Control
Audit preparation for document control should focus on demonstrating system effectiveness through evidence of appropriate document use and control. This includes showing that personnel have access to current documents and that obsolete documents are properly controlled.
Preparation activities include document control system reviews, user access testing, and verification that actual practices align with documented procedures. Internal audits help identify potential issues before external assessment.
ISO 9001 internal audit processes provide structured approaches to audit preparation and system evaluation.
Common Non-Conformities and Solutions
Common non-conformities in document control include the use of obsolete documents, inadequate access controls, missing approval signatures, and failure to maintain documented information currency. Understanding these issues enables proactive prevention.
Solutions focus on system improvements rather than just correcting individual instances. Root cause analysis helps identify underlying system weaknesses that enable recurrence.
Preventive measures include automated controls, regular system reviews, and enhanced training that address systemic issues rather than individual mistakes.
Corrective Action Documentation
Corrective action documentation for document control non-conformities must address both immediate corrections and systemic improvements that prevent recurrence. Effective corrective actions strengthen overall system effectiveness.
Documentation should include problem analysis, root cause identification, corrective actions taken, and verification of effectiveness. This information supports continuous improvement while demonstrating management commitment.
Follow-up activities ensure that corrective actions achieve intended results without creating unintended consequences elsewhere in the quality system.
ISO 9001 Version 2015 Updates for Document Control
ISO 9001 version 2015 introduced significant changes to document control requirements, moving from prescriptive documentation lists to risk-based approaches that enable organizational flexibility while maintaining necessary control.
The transition to "documented information" terminology reflects modern documentation practices while maintaining fundamental control principles that ensure quality system effectiveness.
Changes from Previous Versions
Changes from previous versions include elimination of mandatory quality manual requirements, consolidation of document and record control into unified documented information control, and emphasis on organizational determination of necessary documentation.
These changes enable organizations to optimize their documentation approaches based on their specific context, complexity, and risk profiles rather than following one-size-fits-all requirements.
Organizations transitioning from previous versions should evaluate their current documentation to identify opportunities for simplification and improved effectiveness.
Transition Requirements
Transition requirements focus on ensuring that revised documentation approaches continue to meet quality system effectiveness needs while potentially reducing unnecessary documentation burden.
Transition planning should include documentation review, system updates, personnel training, and gradual implementation that minimizes operational disruption while building user confidence.
ISO 9001 standard update overviews provide additional guidance for managing transitions effectively.
Implementation Timeline Considerations
Implementation timeline considerations include system development, personnel training, pilot testing, and full deployment phases that enable successful adoption without operational disruption.
Realistic timelines account for organizational change management needs, technology implementation requirements, and personnel training needs. Rushed implementations often create more problems than they solve.
Phased approaches enable learning and refinement during implementation while building organizational confidence in new systems and procedures.
How BPRHub Helps with Document Control
BPRHub's comprehensive Quality, Compliance, and Governance platform revolutionizes ISO 9001 document control by centralizing all documented information within an intelligent, automated system that eliminates traditional document management challenges. The platform transforms document control from an administrative burden into an operational competitive advantage.
With automated workflow routing, version control, and access management, BPRHub ensures that personnel always access current, approved, documented information while maintaining complete audit trails that satisfy ISO 9001 certification requirements. The platform's intelligent alerts prevent the use of obsolete documents while streamlining approval processes.
BPRHub's Unified Compliance Framework integrates ISO 9001 documentation requirements with other regulatory obligations, eliminating duplicate workflows while ensuring comprehensive compliance coverage. This integration approach reduces administrative overhead while strengthening overall quality system effectiveness.
The platform's advanced analytics provide insights into document usage patterns, enabling optimization that improves operational efficiency while maintaining robust control capabilities that support continuous audit readiness.
Key Takeaways
→ ISO 9001 Clause 7.5.3 replaces separate document and record control with unified documented information control that provides flexibility while maintaining necessary oversight
→ QMS document control systems must balance accessibility with security, ensuring personnel can efficiently access current information while preventing unauthorized modifications
→ Documentation control systems should leverage digital technologies to provide automated workflows, version control, and comprehensive audit trails that support operational excellence
→ ISO 9001 compliance requires evidence of systematic documented information control throughout the lifecycle from creation through secure disposal
→ ISO 9001 training for document control must address both system operation and quality management principles to ensure effective implementation across all organizational levels
→ BPRHub's platform automates document control complexities while providing intelligent insights that optimize documentation effectiveness and support continuous improvement.
Get audit-ready with ease, explore how BPRHub simplifies Clause 7.5.3 documentation management today.
📍 Book a Demo
📧 hello@bprhub.com
FAQ
Q. What is ISO 9001 Clause 7.5.3 control of documented information?
ISO 9001 Clause 7.5.3 establishes requirements for controlling documented information throughout its lifecycle, from creation to disposal. According to the ISO 9001:2015 standard, this clause requires organizations to ensure documented information is available when needed, suitable for use, and adequately protected. The clause addresses identification, storage, protection, retrieval, retention, and disposition of all documented information required for quality management system effectiveness and evidence of conformity.
Q. What is ISO 27001 Clause 7.5.3 control of documented information?
ISO 27001 Clause 7.5.3 mirrors ISO 9001's approach but focuses specifically on information security management system (ISMS) documentation. According to ISO 27001:2013, this clause requires organizations to control documented information needed for ISMS effectiveness and evidence of conformity. While the structure is similar to ISO 9001, the focus is on security-related documentation, including policies, procedures, and records that support information security objectives and risk management.
Q. What is the ISO 9001 requirement of document control?
ISO 9001 document control requirements ensure that documented information remains current, accessible, and protected throughout its lifecycle. According to ISO 9001:2015, organizations must establish systematic approaches to document identification, distribution, access, retrieval, use, storage, preservation, and disposition. The standard requires that obsolete documents are prevented from unintended use while ensuring authorized personnel have access to current, approved versions. Documentation control systems must demonstrate that documented information supports quality management system effectiveness.
Q. What is the QMS Clause 7.1.3?
QMS Clause 7.1.3 in ISO 9001:2015 addresses infrastructure requirements for quality management systems. According to the standard, organizations must determine, provide, and maintain the infrastructure necessary for process operation and product/service conformity. This includes buildings, workspace, equipment, transportation resources, and information and communication technology. While not directly related to document control, Clause 7.1.3 infrastructure requirements often include document management systems and technology platforms that support documented information control under Clause 7.5.3.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

