Food quality control failures devastate manufacturers across every sector. According to CIDRAP in 2024, there were 296 total recalls, with 1,392 people sickened and hospitalizations more than doubling from 230 in 2023 to 487 in 2024. The Boar's Head deli meat crisis alone caused 61 illnesses and 10 deaths from Listeria contamination, underscoring how critical robust preventive systems have become.
Manufacturers today navigate an increasingly complex regulatory landscape where reactive compliance strategies no longer suffice. Traditional HACCP (Hazard Analysis and Critical Control Points) frameworks, while foundational, require evolution to meet modern risk profiles. HARPC (Hazard Analysis and Risk-Based Preventive Controls) offers a comprehensive approach that transforms food and beverage quality control from reactive monitoring to proactive prevention.
Understanding the fundamental distinctions between these frameworks, regulatory implications, and how manufacturers can leverage unified compliance management enables operational excellence. Whether you're implementing your first HACCP food safety program or navigating HARPC certification requirements, understanding the HARPC vs HACCP differences enables strategic decision-making that protects both consumers and business continuity.
What is HACCP?
HACCP represents the cornerstone of modern food safety management, establishing systematic approaches to hazard identification and control. As a globally recognized risk-based preventative approach recommended by the Codex Alimentarius Commission (CAC), HACCP food safety programs commonly focus on controlling three main food safety hazards: biological, chemical, and physical.
How HACCP Developed
Aerospace industry quality control systems gave birth to HACCP, which adapted systematic hazard analysis for food production environments. The system has been continually developed and updated since its conceptualization in the 1960s, establishing proven methodologies for risk management across diverse manufacturing operations.
What are The Seven HACCP Principles?
Seven fundamental principles guide the framework's operation
Conduct Hazard Analysis: Systematic identification of potential biological, chemical, and physical hazards throughout production processes
Identify Critical Control Points (CCPs): Establish specific stages where control measures prevent, eliminate, or reduce hazards to acceptable levels
Establish Critical Limits: Define measurable parameters that must be met at each CCP to ensure food safety
Establish Monitoring Systems: Implement continuous or batch monitoring procedures to verify CCP performance
Establish Corrective Actions: Define immediate responses when monitoring indicates deviation from critical limits
Establish Verification Procedures: Validate that the HACCP system functions effectively through an independent assessment
Establish Documentation and Record-keeping: Maintain comprehensive records demonstrating system implementation and effectiveness
Read more on What Is HACCP? A Practical Guide to Food Safety & Compliance
Where does HACCP apply?
USDA and FDA only mandate HACCP for meat, seafood, and juice products, though manufacturers widely use the framework due to requirements from retailers, auditing standards, and inspectors. Such selective application creates compliance complexity for manufacturers serving multiple market segments or pursuing diverse standards.
HACCP food safety programs center on Critical Control Points, specific process stages where measurable controls prevent food safety hazards. Each control point must include measurable critical limits, the temperature and length of time a sauce must be held at, for example, as a kill step. Such precision enables manufacturers to establish clear operational parameters while maintaining flexibility across different product lines.
What is HARPC?
HARPC represents a paradigm shift in food safety management, mandated under the Food Safety Modernization Act (FSMA) of 2011. Under the FSMA Act, the FDA now has a legislative mandate to require comprehensive, science-based preventive controls across the food supply chain.
How HARPC Works Differently
Unlike HACCP's focus on process-specific critical control points, HARPC demands comprehensive hazard evaluation across entire operations. HARPC addresses biological, chemical, physical, and radiological hazards, natural toxins, pesticides, drug residues, decomposition, parasites, allergens, unapproved food, and color additives, naturally occurring hazards or unintentionally introduced hazards, and intentionally introduced hazards (including acts of terrorism).
Such expanded scope requires manufacturers to evaluate risks beyond traditional process controls, incorporating supply chain vulnerabilities, facility security, and environmental factors into comprehensive risk assessments.
HARPC Prevention Focus
HARPC emphasizes preventive controls rather than reactive monitoring. HARPC establishes proactive preventive controls that work to prevent hazards in the first place, fundamentally shifting compliance focus from detection to prevention.
Ready to Strengthen Your Food Safety Program? Streamline your HACCP and HARPC requirements today.
📍 Book a Demo
📧 hello@bprhub.com
What are the five HARPC steps involved?
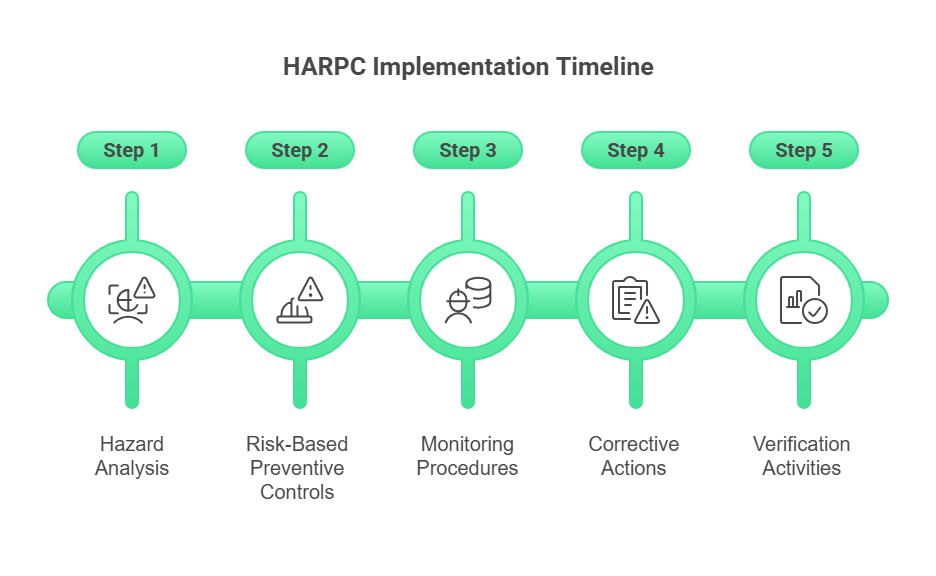
HARPC (Hazard Analysis and Risk-Based Preventive Controls) follows a systematic five-step approach that transforms food safety from reactive monitoring to proactive prevention. Each step builds upon the previous one, creating a comprehensive framework that addresses modern food safety challenges beyond traditional HACCP food safety program requirements.
Step 1: Hazard Analysis
Comprehensive Hazard Identification Process
- Manufacturers conduct facility-wide hazard assessments across all operational areas, including production lines, storage facilities, receiving areas, and packaging operations. Unlike traditional HACCP food safety programs, which focus primarily on biological, chemical, and physical hazards, HARPC requires evaluation of radiological hazards, natural toxins, pesticides, drug residues, allergens, and intentional contamination threats.
- Facilities assess environmental factors, including facility design, air quality, water systems, pest control programs, and personnel practices that could contribute to food safety hazards. They consider seasonal variations, equipment age, and maintenance schedules that might affect hazard likelihood.
- Quality managers assess environmental factors, including facility design, air quality, water systems, pest control programs, and personnel practices that could contribute to food safety hazards. Teams consider seasonal variations, equipment age, and maintenance schedules that might affect hazard likelihood.
- Companies review historical data from customer complaints, internal quality records, supplier audits, and industry recall databases to identify patterns and recurring hazard types specific to their product categories and operational environment.
- Manufacturers document hazard severity and likelihood using risk assessment matrices that consider both the probability of occurrence and potential impact on consumer health, enabling prioritization of preventive control efforts.
Step 2: Risk-Based Preventive Controls
Preventive Control Selection and Implementation
- Companies design targeted preventive controls for each identified hazard using science-based approaches that significantly minimize or prevent hazard occurrence rather than simply detecting problems after they occur. Controls must be specific to the hazard type, product characteristics, and operational environment.
- Manufacturers establish process preventive controls such as heat treatment parameters, pH adjustments, water activity controls, and antimicrobial interventions that directly address biological hazards during production processes.
- Facilities implement allergen preventive controls including segregation procedures, cleaning protocols, labeling verification, and cross-contact prevention measures that protect consumers with food allergies from undeclared allergen exposure.
- Companies deploy sanitation preventive controls covering food contact surfaces, equipment cleaning procedures, environmental monitoring programs, and personnel hygiene requirements that prevent contamination throughout the facility.
- Manufacturers install supply chain preventive controls through supplier verification programs, incoming material inspections, and certificate of analysis requirements that ensure hazard prevention begins before materials enter their facility.
Step 3: Monitoring Procedures
Continuous and Systematic Monitoring Systems
- Companies develop monitoring protocols that verify preventive controls operate as intended through measurable parameters, observation checklists, or testing procedures appropriate to each control type. Monitoring frequency must be sufficient to ensure control effectiveness.
- Manufacturers establish critical parameters for each preventive control, defining acceptable ranges, measurement methods, and monitoring equipment calibration requirements. Parameters should be scientifically validated and directly related to hazard prevention.
- Facilities assign monitoring responsibilities to qualified personnel with appropriate training and authority to take corrective actions when monitoring indicates preventive control deviations. Clear job descriptions and competency requirements ensure consistent monitoring execution.
- Companies implement real-time monitoring systems where feasible, using automated sensors, continuous recording devices, and digital data collection tools that provide immediate feedback on preventive control performance.
- Manufacturers create monitoring documentation that captures all monitoring activities, results, and observations in formats that support regulatory compliance and facilitate trend analysis for continuous improvement.
Step 4: Corrective Actions
Systematic Response to Preventive Control Failures
- Companies establish immediate corrective actions that address the specific deviation and prevent the affected product from entering commerce. Actions must be taken promptly when monitoring indicates preventive controls are not operating as intended.
- Manufacturers develop root cause analysis procedures that identify underlying reasons for preventive control failures, going beyond immediate symptoms to address systemic issues that could lead to recurring problems.
- Facilities implement corrective action protocols that restore preventive control effectiveness and prevent recurrence through equipment repairs, procedure modifications, additional training, or system redesign as appropriate.
- Companies define product disposition procedures for evaluating the safety of products produced during preventive control deviations, including hold, rework, or disposal decisions based on hazard assessment and product safety evaluation.
- Manufacturers document all corrective actions with detailed records of the deviation, investigation findings, actions taken, and verification of effectiveness to demonstrate regulatory compliance and support continuous improvement efforts.
Step 5: Verification Activities
Independent Assessment and Continuous Improvement
- Companies conduct verification activities that confirm preventive controls are consistently implemented and effective in significantly minimizing or preventing identified hazards. Verification must be performed by qualified individuals who are not directly responsible for performing the activities being verified.
- Manufacturers perform calibration and validation of monitoring equipment, testing methods, and measurement systems to ensure the accuracy and reliability of data used to verify preventive control performance.
- Facilities review monitoring records and corrective action documentation to identify trends, assess system effectiveness, and determine whether preventive controls require modification or enhancement.
- Companies conduct environmental monitoring where appropriate to verify that sanitation preventive controls effectively prevent contamination of food contact surfaces and the production environment.
- Manufacturers implement reanalysis requirements that mandate a comprehensive review of the entire food safety plan at least once every three years or when significant changes occur that could affect hazard analysis or preventive control adequacy.
- Companies validate preventive control measures through scientific studies, challenge testing, or other appropriate methods that demonstrate the controls achieve their intended food safety outcomes under actual operating conditions.
What are the HARPC Requirements?
A HACCP food safety program is not mandatory, but FSMA HARPC is mandated under law. Such regulatory distinction creates significant compliance obligations for manufacturers falling under FDA jurisdiction.
HARPC certification requires qualified personnel oversight through Preventive Controls Qualified Individuals (PCQIs), who must demonstrate competency in food safety system development, implementation, and maintenance. A "qualified individual" or a "team of qualified individuals" from a facility is required to understand significant food safety hazards and put in place preventive controls to minimize the risk of hazards.
What are the Key Differences Between HACCP and HARPC?
Regulatory Scope
The fundamental difference between frameworks lies in regulatory applicability and scope. Both HACCP and HARPC are food safety standards, but HARPC covers food safety concerns beyond CCPs and is mandated for most facilities under FDA oversight, with some exemptions, while HACCP maintains a specific industry focus.
HACCP food safety programs apply primarily to meat, seafood, and juice processing operations under USDA and FDA oversight. Conversely, HARPC encompasses nearly all FDA-regulated food facilities, creating broader compliance obligations across diverse manufacturing sectors.
Hazard Analysis Approach
HACCP food safety programs concentrate on critical control points within established processes, emphasizing measurable controls at specific stages. Critical Control Points during process steps are central to HACCP, with each control point including measurable critical limits.
HARPC adopts a holistic approach, evaluating all reasonably foreseeable hazards regardless of occurrence within traditional process controls. During hazard analysis, HACCP food safety programs focus on biological, chemical, and physical hazards, while HARPC includes a wider range of potential hazards, such as radiological hazards, natural toxins, parasites, and intentional adulteration.
Documentation Requirements
HARPC certification demands more comprehensive documentation than traditional HACCP food safety programs. HARPC standards typically require more detailed documentation and should be reviewed once every three years, compared to HACCP's annual review requirements.
Such documentation intensity reflects HARPC's comprehensive approach to risk management, requiring manufacturers to maintain detailed records of hazard analyses, preventive control justifications, monitoring procedures, and verification activities.
Personnel Qualifications
HACCP food safety program implementation typically involves multidisciplinary teams with designated coordinators responsible for system oversight. In HACCP, the responsibility for implementing and maintaining the system typically falls on the HACCP coordinator, who ensures that all principles are followed.
HARPC certification introduces specific qualification requirements through PCQIs, who must demonstrate competency in food safety system development and maintenance. In HARPC, the task is assigned to a Preventive Controls Qualified Individual (PCQI), establishing clear accountability for system effectiveness.
Who Needs HARPC?
Any facility that manufactures, processes, packs, distributes, receives, holds or imports food must develop a HARPC plan for compliance with FSMA HARPC requirements.
Except as exempted below, all facilities subjected to the FDA's Bioterrorism Facility Establishment registration, both in the United States and abroad, that are producing food products for distribution in the United States must develop and implement a HARPC plan.
Who is Exempted from HARPC?
Several categories are exempted from the HARPC plan requirement:
- Facilities under USDA jurisdiction handling, processing, and shipping meat, poultry, eggs, etc.
- Operations under the FDA's Seafood and Juice HACCP regulations
- Facilities subject to the FDA's new standards for Produce Safety Authorities
- Low-acid and acidified canned food processors
- Facilities defined as "small" or "very small" businesses
- Facilities with a previous 3-year average product value or revenue of less than $500,000
Such exemptions create compliance complexity for manufacturers operating across multiple categories or serving diverse market segments, requiring careful evaluation of applicable requirements.
Do HACCP and HARPC Work Together?
Rather than viewing frameworks as competing systems, manufacturers benefit from understanding the complementary relationship. A HARPC plan shouldn't be considered as a replacement but as a necessary upgrade to the conventional HACCP food safety program.
Since the passage of the Food Safety Modernization Act (FSMA) in 2011, there has been a need for a food safety plan that meets the preventive controls for human food and the typical Hazard Analysis and Critical Control Points (HACCP) requirements. Such integration challenge requires systematic approaches that address both frameworks' requirements without duplicating efforts.
What are the Implementation Challenges?
Important differences between the rules of the preventive controls regulations and the principles of HACCP make meeting both sets of requirements difficult. Manufacturers face significant challenges in developing integrated systems that satisfy both frameworks without excessive duplication.
Successful implementation requires comprehensive planning, qualified personnel, and systematic approaches to hazard analysis that address both HACCP food safety program process-focused controls and HARPC's comprehensive prevention requirements.
How BPR Hub Helps with Both Systems
Managing dual compliance requirements presents operational complexity that traditional document-based systems cannot effectively address. BPR Hub's Unified Compliance Framework eliminates challenges through integrated management capabilities that streamline both HACCP and HARPC requirements.
Centralized Management
BPR Hub consolidates hazard analysis activities for both frameworks within a single platform, enabling manufacturers to conduct comprehensive evaluations that satisfy HACCP's CCP requirements while addressing HARPC's expanded hazard categories. The platform maintains complete audit trails, version control, and regulatory documentation required for both systems.
Automated Controls
The platform automates preventive control implementation and monitoring, tracking both HACCP food safety program critical limits and HARPC preventive control parameters through real-time dashboards. Such a dual-system approach ensures compliance with both frameworks while eliminating duplicate monitoring efforts.
Training Management
BPR Hub streamlines personnel qualification requirements, managing both HACCP food safety program team competencies and HARPC certification PCQI standards through integrated training platforms. The system tracks qualification maintenance, renewal requirements, and competency assessments for both frameworks.
Audit Preparation
The platform generates comprehensive verification documentation that demonstrates compliance with both HACCP food safety program principles and HARPC requirements. Automated audit packages include hazard analyses, control records, verification activities, and corrective action documentation required for regulatory inspections
Don’t Wait for the Next Recall! Upgrade your food safety plan with BPR Hub today
📍 Book a Demo
📧 hello@bprhub.com
FAQ
Q. What is the difference between HACCP and HARPC?
HACCP vs HARPC differ primarily in their scope and hazard definitions. HACCP defines hazards as chemical, physical, or biological agents likely to cause illness, while HARPC defines hazards as known or reasonably foreseeable food safety risks that may cause illness if present in food products. HARPC is considered an upgraded version of HACCP because it covers broader food safety concerns beyond Critical Control Points (CCPs) and includes additional risks like natural allergens, radiological hazards. While HACCP focuses on contaminant agents, HARPC addresses potential risks throughout the entire supply chain. HARPC is mandated by the FDA for most facilities, whereas HACCP is primarily required for meat, seafood, and juice products.
Q. What replaced HACCP?
HACCP was not completely replaced but rather enhanced by HARPC (Hazard Analysis and Risk-Based Preventive Controls) under the Food Safety Modernization Act. HARPC should be considered as a necessary upgrade to conventional HACCP food safety programs rather than a replacement. The two systems work together, with HARPC providing a broader approach that encompasses additional food safety requirements beyond traditional HACCP principles. HARPC includes preventive controls that may be applied at CCPs along with other control measures throughout the food production process. While HACCP remains widely used due to retailer requirements and auditing standards, HARPC adds mandatory compliance for FDA-regulated facilities.
Q. What are the 7 principles of HACCP?
The HACCP food safety program is built on seven fundamental principles that ensure comprehensive hazard control.
Principle 1: Conduct a hazard analysis to identify biological, chemical, and physical hazards in the production process.
Principle 2: Determine Critical Control Points (CCPs) where hazards can be prevented, eliminated, or reduced to acceptable levels.
Principle 3: Establish critical limits - maximum or minimum values that must be met at each CCP based on scientific findings.
Principle 4: Establish monitoring procedures to measure and observe whether critical limits are being met.
Principle 5: Establish corrective actions when critical limits are not met to bring CCPs back into control.
Principle 6: Establish verification procedures to confirm the HACCP system is working properly.
Principle 7: Establish recordkeeping procedures to document plan implementation and provide proof of regulatory compliance.
Q. What is HARPC in the food industry?
HARPC (Hazard Analysis and Risk-Based Preventive Controls) is a science-based food safety system mandated by the FDA under the Food Safety Modernization Act for most food processing facilities. HARPC requires facilities to conduct comprehensive risk assessments to identify and prevent potential hazards in their operations, including those that can naturally occur or be unintentionally introduced. The system encompasses broader food safety assessment and control measures compared to traditional HACCP plans, addressing hazards throughout the entire supply chain. HARPC certification involves rigorous training and assessment, with courses covering hazard analysis, preventive controls, monitoring, verification procedures, recall plans, and recordkeeping.
Q. What are the three types of HACCP?
HACCP addresses three main types of hazards rather than three types of HACCP systems: biological, chemical, and physical hazards.
Biological hazards include microorganisms such as bacteria, viruses, yeasts, molds, and parasites that can cause foodborne illness, with examples including Salmonella, E. coli, and Clostridium botulinum.
Chemical hazards encompass various substances, like improper pesticide use, antimicrobial residues, processing chemicals, sanitizers, and substances that become dangerous at high concentrations, such as sodium nitrite.
Physical hazards include hard or sharp objects like glass, metal, plastic, stones, wood, or bone that can cause injuries such as choking, cuts, or broken teeth. These three hazard categories form the foundation of any HACCP food safety program, requiring systematic identification, assessment, and control measures throughout the food production process.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

