Life sciences companies face increasingly stringent regulatory oversight, with GXP compliance violations resulting in billions of dollars in FDA fines annually. According to FDA enforcement statistics, over 60% of warning letters issued to pharmaceutical companies cite GXP requirements failures, leading to production shutdowns and market delays.
What is GXP, and why does it matter? GXP represents a comprehensive framework of pharmaceutical regulatory compliance standards that ensure product quality, patient safety, and data integrity across the entire life sciences value chain. Modern GXP compliance transforms these regulatory requirements from administrative burdens into competitive advantages through systematic implementation and intelligent automation.
This definitive guide explores how GXP guidelines create operational excellence while maintaining regulatory adherence across pharmaceutical manufacturing compliance operations.
What is GXP Compliance and Why Does It Matter
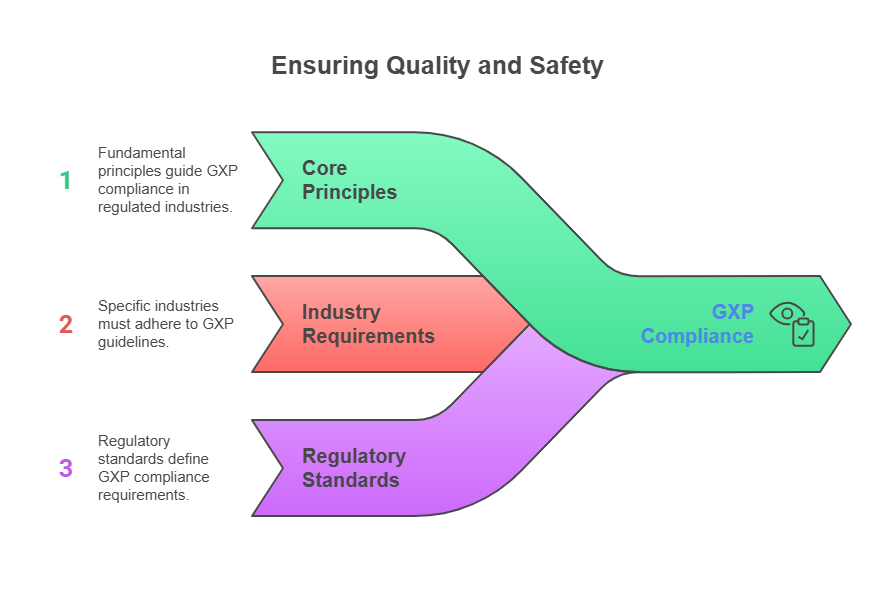
GXP compliance encompasses a family of quality assurance regulations that govern life sciences industries worldwide. The "X" in GXP represents various practice areas, including Good Manufacturing Practices (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP), each addressing specific aspects of pharmaceutical development and manufacturing.
These regulations ensure that pharmaceutical products meet safety, efficacy, and quality requirements throughout their lifecycle. GXP compliance protects patients while enabling companies to maintain market access across global markets.
Definition of GXP Standards in Regulated Industries
GXP standards establish systematic approaches to quality management that span from research and development through commercial manufacturing. These standards require organizations to implement comprehensive quality management systems that ensure consistent product quality and regulatory adherence.
The framework emphasizes documentation, training, validation, and continuous monitoring to maintain product integrity throughout the supply chain. GXP standards create structured environments where quality decisions are based on scientific evidence and regulatory requirements.
Modern GXP compliance implementations leverage technology to automate routine compliance tasks while providing real-time visibility into quality management metrics across all operations.
Core Principles Behind GXP Regulations
GXP regulations are built on fundamental principles that prioritize patient safety, product quality, and data integrity. These principles include proper personnel training, adequate facility design, validated processes, and comprehensive documentation systems.
The regulations emphasize risk-based approaches that focus resources on activities with the greatest impact on product quality and patient safety. This systematic methodology enables organizations to optimize compliance investments while maintaining regulatory standards.
Quality assurance principles embedded within GXP regulations require organizations to implement preventive measures rather than relying solely on final product testing to ensure quality.
Industries Required to Follow GXP Guidelines
GXP guidelines apply across multiple life sciences sectors, including pharmaceuticals, biotechnology, medical devices, and clinical research organizations. Each industry faces specific regulatory requirements tailored to its unique risk profiles and patient safety considerations.
Pharmaceutical manufacturers must comply with comprehensive GMP compliance requirements that address facility design, personnel training, process validation, and product release procedures. Medical device companies follow similar principles adapted for device-specific manufacturing processes.
Clinical research organizations implement Good Clinical Practice standards to ensure trial data integrity and patient safety throughout clinical development programs.
Essential GXP Requirements Every Organization Must Meet
GXP requirements establish systematic frameworks for maintaining product quality and regulatory compliance across all life sciences operations. These requirements address personnel qualifications, facility standards, documentation practices, and equipment validation procedures.
Organizations must demonstrate systematic implementation of quality management systems that address all aspects of their operations while maintaining continuous compliance monitoring and improvement programs.
Quality Assurance Fundamentals in GXP
Quality assurance forms the foundation of GXP compliance by establishing systematic approaches to preventing quality issues before they occur. These programs require organizations to implement comprehensive quality management systems that address all aspects of their operations.
The quality assurance framework includes personnel training programs, facility qualification procedures, equipment validation protocols, and document management systems. These elements work together to create comprehensive quality environments that ensure consistent product quality.
Modern quality assurance implementations leverage quality management software to automate routine quality tasks while providing real-time visibility into quality metrics across all manufacturing operations.
Documentation Standards and Record-Keeping
Documentation requirements under GXP compliance mandate comprehensive record-keeping that demonstrates systematic implementation of quality procedures. These records must be contemporaneous, complete, and attributable to specific individuals and time periods.
Electronic documentation systems must comply with FDA 21 CFR Part 11 requirements for electronic records and signatures, ensuring data integrity and audit trail completeness.
Effective document management systems provide version control, automated workflow routing, and comprehensive audit trails that satisfy regulatory inspection requirements while streamlining operational efficiency.
Personnel Training and Qualification Requirements
Personnel training under GXP requirements ensures that all individuals involved in regulated activities possess appropriate qualifications and maintain current competencies. Training programs must address both technical skills and regulatory requirements specific to each role.
Training management systems automate training delivery, track completion status, and maintain comprehensive training records that demonstrate ongoing personnel qualification.
Regular competency assessments ensure that personnel maintain appropriate skill levels while identifying additional training needs that support continuous improvement initiatives.
Equipment Validation and Maintenance
Equipment validation ensures that manufacturing and testing equipment operate consistently within predetermined parameters throughout their operational life. Validation protocols must demonstrate that equipment meets user requirements and regulatory standards.
Preventive maintenance programs maintain equipment performance while preventing quality issues that could impact product safety or efficacy. These programs include regular calibration, performance verification, and predictive maintenance activities.
Quality control procedures verify that equipment continues to operate within validated parameters while identifying potential issues before they impact product quality or manufacturing operations.
Streamline Your GXP Compliance Today with BPRHub – Stay Audit-Ready, Always.
📍 Book a Demo
📧 hello@bprhub.com
How Good Manufacturing Practices serve as the Foundation of GXP
Good manufacturing practices establish comprehensive requirements for pharmaceutical manufacturing that ensure consistent product quality and regulatory compliance. These standards address facility design, personnel training, process validation, and quality control procedures.
GMP compliance creates systematic manufacturing environments where quality is built into processes rather than inspected into final products. This approach reduces manufacturing risks while improving operational efficiency.
GMP Compliance Requirements for Production Facilities
GMP compliance requirements mandate specific facility design features that support pharmaceutical manufacturing while maintaining product quality and preventing contamination. These requirements address air handling systems, water systems, and material flow patterns.
Facility qualification protocols demonstrate that manufacturing environments consistently maintain appropriate conditions for pharmaceutical production. These protocols include installation qualification, operational qualification, and performance qualification phases.
Environmental monitoring programs verify that manufacturing areas maintain appropriate conditions while identifying potential sources of contamination that could impact product quality.
Quality Control Procedures in Manufacturing
Quality control procedures verify that raw materials, in-process materials, and finished products meet predetermined specifications before release for distribution. These procedures include sampling plans, test methods, and acceptance criteria.
Laboratory testing must follow validated analytical methods that demonstrate accuracy, precision, and reliability for intended applications. Method validation protocols ensure that test results accurately reflect product quality characteristics.
Quality control data management systems maintain complete testing records while providing trending capabilities that identify potential quality issues before they impact manufacturing operations.
Facility Design and Environmental Controls
Facility design under GMP compliance requirements ensures that manufacturing areas support product quality while preventing contamination and cross-contamination. Design features include appropriate air handling, water systems, and material flow patterns.
Environmental control systems maintain temperature, humidity, and air quality within predetermined ranges while providing continuous monitoring capabilities. These systems include backup capabilities that prevent environmental excursions during equipment failures.
Cleaning and sanitization procedures maintain hygienic conditions throughout manufacturing areas while preventing cross-contamination between different products or manufacturing campaigns.
Raw Material Management and Testing
Raw material management ensures that incoming materials meet quality specifications while maintaining complete traceability throughout the manufacturing process. Material qualification procedures verify supplier capabilities while establishing ongoing monitoring programs.
Incoming material testing verifies that raw materials meet predetermined specifications before release for manufacturing use. Testing programs include identity, purity, and potency testing appropriate for intended applications.
Material storage and handling procedures maintain material quality while preventing contamination or degradation during storage periods. These procedures include environmental controls, container integrity monitoring, and inventory management systems.
Production Process Validation
Process validation demonstrates that manufacturing processes consistently produce products that meet predetermined quality attributes. Validation protocols include process design, process qualification, and continued process verification phases.
Process monitoring systems capture real-time manufacturing data while identifying trends that could indicate process drift or potential quality issues. These systems provide early warning capabilities that enable proactive interventions.
Statistical process control methodologies identify process variations while distinguishing between common cause and special cause variations that require different response strategies.
Finished Product Release Procedures
Product release procedures ensure that finished products meet all quality specifications before distribution to patients or healthcare providers. These procedures include final product testing, batch record review, and disposition decision processes.
Batch disposition decisions must be made by qualified individuals who review all manufacturing and testing data to ensure product quality and regulatory compliance. These decisions must be documented with appropriate justifications.
Product distribution systems maintain product integrity during storage and transportation while providing complete traceability throughout the distribution network.
Good Clinical Practice Standards for Research
Good clinical practice standards ensure that clinical trials are conducted ethically while generating reliable data that supports regulatory submissions. These standards address trial design, conduct, monitoring, and data management procedures.
GCP compliance protects trial participants while ensuring data integrity throughout clinical development programs. These standards apply to all individuals and organizations involved in clinical research activities.
Good Laboratory Practice for Testing and Analysis
Good laboratory practice standards ensure that laboratory testing generates reliable, reproducible results that support regulatory decision-making. These standards address laboratory organization, personnel qualifications, facility requirements, and quality assurance procedures.
GLP compliance creates controlled laboratory environments where testing follows standardized procedures while maintaining complete documentation of all testing activities.
Quality Management Systems for GXP Implementation
Quality management systems provide comprehensive frameworks for implementing GXP compliance requirements across life sciences organizations. These systems integrate quality assurance procedures with operational activities while maintaining continuous compliance monitoring.
Effective quality management systems balance regulatory compliance requirements with operational efficiency while supporting continuous improvement initiatives that enhance both quality and productivity.
What are Industry-Specific GXP Applications
GXP pharma applications address the unique requirements of different life sciences sectors while maintaining common quality management principles that ensure product quality and patient safety across all applications.
Industry-specific requirements reflect different risk profiles and regulatory frameworks while maintaining core GXP compliance principles that ensure systematic quality management implementation.
Medical Device Compliance Requirements
Medical device compliance requirements address the unique characteristics of medical devices while implementing quality management systems that ensure device safety and effectiveness throughout their lifecycle.
Medical device quality management systems must comply with ISO 13485 requirements while maintaining GXP compliance for regulated activities, including clinical trials and manufacturing operations.
Device traceability requirements ensure complete accountability from raw materials through patient use while maintaining comprehensive documentation that supports post-market surveillance activities.
Pharmaceutical Manufacturing Standards
Pharmaceutical manufacturing standards implement comprehensive GMP compliance requirements that address facility design, process validation, quality control, and product release while ensuring consistent product quality.
Manufacturing systems must demonstrate process capability while maintaining statistical process control that identifies potential quality issues before they impact product quality or patient safety.
Batch manufacturing procedures ensure consistent product quality while maintaining complete documentation that supports product release and regulatory compliance requirements.
Biotechnology and Biologics Compliance
Biotechnology and biologics compliance addresses the unique requirements of biological products while implementing GXP compliance requirements that ensure product safety and efficacy.
Biological product manufacturing requires specialized environmental controls and quality control procedures that address the unique characteristics of living systems used in production processes.
Cell bank management and characterization procedures ensure consistent starting materials while maintaining comprehensive documentation that supports product consistency and regulatory compliance.
Contract Manufacturing Organization (CMO) Requirements
CMO requirements ensure that contract manufacturing activities maintain GXP compliance while providing appropriate oversight and quality assurance throughout contracted manufacturing operations.
Quality agreements define responsibilities and requirements while ensuring that CMO activities maintain GMP compliance and support sponsor regulatory compliance obligations.
Supplier qualification procedures ensure that CMOs possess appropriate capabilities while maintaining ongoing monitoring that verifies continued compliance with GXP requirements.
Supply Chain Management in GXP
Supply chain management under GXP compliance ensures that all suppliers and service providers maintain appropriate quality management systems while supporting overall product quality and regulatory compliance.
Supplier qualification procedures assess supplier capabilities while implementing ongoing monitoring programs that verify continued compliance with GXP requirements throughout supplier relationships.
Material traceability systems maintain complete accountability for all materials used in regulated activities while supporting investigation and recall activities when quality issues are identified.
Compliance Management System Implementation
Compliance management systems provide comprehensive frameworks for implementing GXP compliance across life sciences organizations while supporting continuous compliance monitoring and improvement activities.
These systems integrate regulatory requirements with operational activities while providing real-time visibility into compliance status and supporting proactive compliance management strategies.
Building an Effective Compliance Management Software Framework
Compliance management software frameworks provide systematic approaches to GXP compliance implementation while supporting organizational scalability and regulatory change management throughout system operation.
Framework implementation addresses organizational structure, process integration, and technology capabilities while ensuring alignment with GXP requirements and business objectives.
System architecture provides scalable platforms that support organizational growth while maintaining GXP compliance throughout expanding operations and evolving regulatory requirements.
Training Programs and Personnel Development
Training programs ensure that personnel possess appropriate qualifications while maintaining current competencies in GXP compliance requirements and operational procedures.
Comprehensive training programs address both initial qualification and ongoing competency maintenance while providing documentation that supports regulatory compliance and internal quality assurance requirements.
Competency assessment procedures verify training effectiveness while identifying additional training needs that support continuous improvement and career development objectives.
Internal Audit and Inspection Readiness
Internal audit programs provide a systematic assessment of GXP compliance implementation while identifying potential improvement opportunities that strengthen the overall compliance posture.
Audit procedures address all aspects of GXP compliance while providing an objective assessment of system effectiveness and regulatory adherence throughout organizational operations.
Inspection readiness procedures ensure that organizations maintain continuous audit readiness while providing appropriate documentation and personnel preparation for regulatory inspections.
Vendor Qualification and Management
Vendor qualification ensures that suppliers and service providers possess appropriate quality management systems while supporting overall GXP compliance throughout supplier relationships.
Qualification procedures assess vendor capabilities while implementing ongoing monitoring programs that verify continued compliance with GXP requirements and performance standards.
Vendor management systems maintain comprehensive supplier information while providing ongoing performance monitoring that supports supplier relationship management and continuous improvement.
Deviation Management and Investigation
Deviation management systems provide systematic approaches to identifying, investigating, and resolving departures from established procedures while implementing appropriate corrective and preventive actions.
Investigation procedures identify root causes while ensuring that corrective actions address immediate issues and preventive actions prevent similar occurrences throughout ongoing operations.
Deviation trending identifies systemic issues while supporting continuous improvement initiatives that strengthen overall GXP compliance and operational performance.

What are Common GXP Compliance Challenges and Solutions
GXP compliance implementation faces common challenges, including resource constraints, technology integration difficulties, and personnel training requirements that require systematic approaches to successful resolution.
Understanding these challenges enables organizations to develop proactive strategies that prevent compliance issues while supporting operational excellence and regulatory adherence.
Documentation and Record-Keeping Issues
Documentation challenges include maintaining current procedures, ensuring document accessibility, and providing comprehensive audit trails that satisfy regulatory requirements throughout document lifecycles.
Solutions include implementing comprehensive document management systems that automate document lifecycles while providing version control and audit trail capabilities.
Electronic document systems eliminate paper-based vulnerabilities while improving document accessibility and reducing the administrative burden associated with manual document management.
Personnel Training and Competency Gaps
Training challenges include ensuring personnel possess appropriate qualifications while maintaining current competencies in evolving GXP requirements and technological capabilities.
Solutions include implementing comprehensive training management systems that automate training delivery while providing competency tracking and assessment capabilities.
Ongoing training programs address both regulatory requirements and operational needs while supporting career development and organizational capability enhancement.
Technology Integration Difficulties
Technology integration challenges include connecting multiple systems while maintaining data integrity and FDA 21 CFR Part 11 compliance throughout integrated system operations.
Solutions include implementing unified platforms that provide comprehensive GXP compliance capabilities while supporting integration with existing operational systems through validated interfaces.
BPRHub's platform addresses integration challenges by providing comprehensive quality management systems that integrate seamlessly with existing organizational infrastructure.
Regulatory Change Management
Regulatory change management challenges include monitoring evolving requirements while implementing necessary procedural updates that maintain compliance with new regulatory expectations.
Solutions include implementing change management systems that monitor regulatory updates while providing systematic approaches to assessing change impacts and implementing necessary modifications.
Automated compliance monitoring provides early warning of regulatory changes while supporting proactive compliance management strategies that prevent compliance gaps.
Cost Management and Resource Allocation
Cost management challenges include balancing compliance investments with operational requirements while ensuring adequate resources for GXP compliance maintenance and improvement activities.
Solutions include implementing risk-based approaches that focus resources on activities with the greatest impact on product quality and regulatory compliance while optimizing compliance investments.
Technology solutions provide cost-effective compliance capabilities while reducing manual administrative burden and improving compliance consistency throughout organizational operations.
What are GXP Compliance Best Practices and Implementation
GXP compliance best practices provide systematic approaches to implementing comprehensive quality management systems that ensure regulatory adherence while supporting operational excellence and business growth.
These practices address organizational culture, continuous improvement, and performance monitoring while providing frameworks for sustainable compliance management throughout organizational evolution.
Establishing a Compliance Culture
Compliance culture establishment requires leadership commitment while implementing systematic approaches that integrate GXP compliance requirements with daily operational activities and decision-making processes.
Cultural transformation addresses personnel attitudes and behaviors while providing training and communication programs that reinforce the importance of quality assurance and regulatory compliance.
Management commitment demonstrates organizational priorities while providing necessary resources and support for comprehensive GXP compliance implementation and maintenance.
Continuous Improvement Programs
Continuous improvement programs identify opportunities for enhancing GXP compliance while supporting operational efficiency and quality management throughout organizational activities.
These programs include regular system assessments while implementing data-driven improvement initiatives that strengthen compliance posture and operational performance.
Performance monitoring provides ongoing visibility into compliance effectiveness while supporting proactive management strategies that prevent compliance issues and support business objectives.
Regulatory Inspection Preparation
Inspection preparation ensures that organizations maintain continuous audit readiness while providing comprehensive documentation and personnel preparation for regulatory inspections.
Preparation activities include regular self-assessments while implementing corrective actions that address potential compliance gaps before regulatory inspections occur.
Inspection procedures provide systematic approaches to inspection management while ensuring appropriate personnel support and documentation availability throughout inspection activities.
Performance Metrics and KPIs
Performance metrics provide a quantitative assessment of GXP compliance effectiveness while supporting data-driven management decisions and continuous improvement initiatives.
Key performance indicators address compliance activities, quality assurance performance, and operational efficiency while providing trending capabilities that identify potential improvement opportunities.
Metrics reporting provides regular visibility into compliance status while supporting management review and strategic planning activities that align compliance with business objectives.
Third-Party Audits and Assessments
Third-party audits provide an objective assessment of GXP compliance implementation while identifying potential improvement opportunities that strengthen overall compliance posture and organizational capabilities.
Audit programs address all aspects of quality management systems while providing independent verification of compliance effectiveness and regulatory adherence.
Assessment results support continuous improvement initiatives while providing external perspectives that enhance organizational quality management capabilities and compliance sustainability.
How BPRHub Helps with GXP Compliance
BPRHub's comprehensive Quality, Compliance, and Governance platform revolutionizes GXP compliance management by centralizing all regulatory requirements within a unified, intelligent system. The platform transforms complex pharmaceutical compliance from fragmented processes into streamlined operational excellence.
With automated quality management systems, real-time compliance monitoring, and integrated document management capabilities, BPRHub ensures your life sciences operations maintain continuous GXP compliance while accelerating product development timelines. The platform's Unified Compliance Framework manages multiple GXP requirements simultaneously, eliminating duplicate workflows that traditionally consume valuable resources.
BPRHub's intelligent automation captures quality control data in real-time, generates regulatory reports automatically, and provides predictive analytics that identify potential compliance issues before they impact operations. This proactive approach reduces regulatory risk while freeing your team to focus on innovation and market expansion.
The platform's validation capabilities ensure FDA 21 CFR Part 11 compliance while providing comprehensive audit trails that satisfy regulatory inspection requirements throughout your quality management processes.
Boost Quality and Cut Compliance Risks – Power Your Operations with BPRHub
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ GXP compliance encompasses multiple practice areas, including GMP, GCP, and GLP, that ensure product quality and patient safety throughout life sciences operations
→ Quality management systems provide comprehensive frameworks for implementing GXP requirements while supporting operational efficiency and regulatory adherence
→ FDA 21 CFR Part 11 compliance ensures electronic systems provide equivalent security and reliability as traditional paper-based documentation systems
→ Document management systems serve as the foundation for GXP compliance by maintaining current procedures and comprehensive audit trails throughout document lifecycles
→ Technology solutions transform manual compliance processes into automated operations that ensure continuous regulatory adherence while reducing administrative burden
→ BPRHub's platform centralizes GXP compliance management while providing intelligent automation that prevents compliance issues before they impact business operations
FAQ
Q. What are the main types of GXP compliance?
The main types of GXP compliance include Good Manufacturing Practices (GMP) for pharmaceutical manufacturing, Good Clinical Practice (GCP) for clinical trials, Good Laboratory Practice (GLP) for testing operations, Good Distribution Practice (GDP) for supply chain, and Good Pharmacovigilance Practice (GVP) for safety monitoring. Each type addresses specific aspects of pharmaceutical development and manufacturing while maintaining common quality assurance principles that ensure product quality and patient safety throughout the product lifecycle.
Q. How do you implement a quality management system for GXP?
Implementing a quality management system for GXP requires systematic planning that addresses organizational structure, process mapping, document management, personnel training, and technology integration. The implementation follows risk-based approaches that focus resources on activities with the greatest impact on product quality while ensuring comprehensive coverage of all GXP requirements. Successful implementation includes stakeholder engagement, change management, and continuous improvement programs that support long-term sustainability and regulatory compliance.
Q. What are the requirements for electronic records under 21 CFR Part 11?
FDA 21 CFR Part 11 requirements for electronic records include validation of computer systems, implementation of appropriate access controls, comprehensive audit trails, electronic signature capabilities, and data backup and recovery procedures. Systems must demonstrate equivalent security and reliability as paper records while maintaining data integrity throughout the record lifecycle. Organizations must establish procedures for system validation, user training, and ongoing compliance monitoring that ensure continued regulatory adherence.
Q. How does GXP compliance differ across industries?
GXP compliance requirements vary across industries based on specific risk profiles and regulatory frameworks while maintaining core quality management principles. Pharmaceutical manufacturing emphasizes GMP compliance with comprehensive facility and process requirements, medical devices focus on ISO 13485 integration with GXP requirements, biotechnology addresses unique biological product characteristics, and clinical
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

