When you look at the numbers, Global food trade reached $1.8 trillion in 2023, with the United States accounting for nearly 10% of worldwide food exports according to the USDA Economic Research Service. Food manufacturers face increasing complexity when securing FDA export certificates to access international markets, particularly as foreign governments tighten import requirements for American food products.
The regulatory landscape governing FDA export certificate procedures for food products has undergone significant evolution, establishing enhanced compliance mechanisms that facilitate international product distribution while maintaining stringent regulatory oversight. Understanding the comprehensive FDA certification framework represents a critical component of successful export operations under current Federal Food, Drug, and Cosmetic Act provisions. In this blog, we see what an FDA export certificate is, the steps to get an FDA certificate, the cost and structuring, and how the BPR Hub helps in managing the FDA certificate.
Understanding FDA Export Certificate Requirements for Food Products
Think of the Food and Drug Administration certificate as your product's official government ID card – it confirms your product's regulatory standing within the United States. Your manufacturing facility needs to tick certain boxes before you can get these certificates, starting with proper facility registration with the FDA and following Current Good Manufacturing Practices (cGMP).
Products that require FDA export certification include
- Processed foods
- Dietary supplements
- Infant formulas
- Food additives
Each product category has its compliance framework. Manufacturers must be able to demonstrate adherence to relevant FDA regulations through well-maintained documentation and up-to-date facility inspection records. These documents help foreign authorities assess the product’s manufacturing environment, safety, and compliance track record. Exporting without this certification significantly limits market access opportunities.
Read more on- How to Get FDA Approval: Process, Standards, and Benefits for Manufacturers
What are the Types of FDA Certification for Export Operations
Certificate to a Foreign Government (CFG) for FDA Export Certificate Applications
The CFG represents the primary FDA export certificate mechanism for conventional foods, food additives, food contact substances, and infant formula products that satisfy applicable Federal Food, Drug, and Cosmetic Act requirements for domestic marketing authorization. This certification provides official attestation that covered products may be marketed within the United States and legally exported under current regulatory provisions.
Regulatory Scope:
- Conventional food products meeting domestic marketing standards
- Food additives with established regulatory approval
- Food contact substances under CFSAN jurisdiction
- Infant formula products comply with specialized regulations
Certificate of Exportability (COE) for Specialized FDA Certification Requirements
Transitioning to export-only products, the COE addresses items that cannot be marketed domestically but satisfy section 801(e)(1) requirements of the Federal Food, Drug, and Cosmetic Act for legal exportation. This FDA certification mechanism facilitates international trade in products manufactured specifically for foreign market compliance while ensuring regulatory compliance standards are maintained.
Certificate of Free Sale (COFS) for Dietary Supplement Food and Drug Administration Certificate Applications
Building upon the certification types, the COFS provides FDA export certificate documentation for dietary supplements, medical foods, and foods for special dietary use without making regulatory status determinations regarding products or manufacturing establishments. This certification acknowledges product existence without regulatory endorsement, facilitating international trade while maintaining regulatory neutrality.
How to Get an FDA Export Certificate
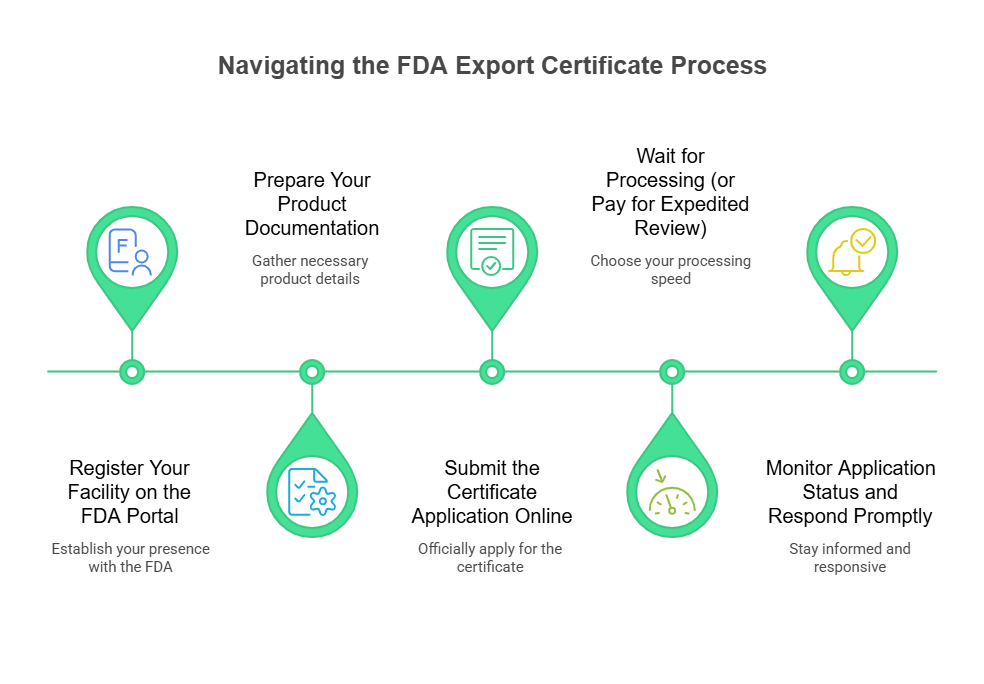
Step 1: Register Your Facility on the FDA Portal
Start by registering your manufacturing facility through the FDA’s online portal to secure your FDA export certificate. Fill out Form FDA 2541 to add your facility to the FDA database. You’ll need to provide detailed information about your operations, including the types of products you make and your contact details.
Step 2: Prepare Your Product Documentation
Once registration is approved, compile full documentation for your product. This includes:
- Complete ingredient lists
- Manufacturing processes
- Quality control procedures
Make sure everything aligns with FDA requirements based on your product type, whether that’s processed foods, beverages, or specialty dietary items.
Step 3: Submit the Certificate Application Online
Use the FDA’s electronic system to apply for your export certificate. Upload all required supporting documents and pay the associated fees.
Step 4: Wait for Processing (or Pay for Expedited Review)
Processing time generally ranges from 20 to 60 business days, depending on application complexity and FDA workload. Need it faster? Expedited processing is available for an additional fee.
Step 5: Monitor Application Status and Respond Promptly
Log in to the FDA portal regularly to check your application status. If the FDA asks for more details, respond immediately. Incomplete applications often get delayed, so having all your materials ready upfront is key to staying on track.
FDA Certification Cost Structure and Processing Timeline
Processing Timeline by Service Type
Streamline your FDA certification process—reduce delays by 60% with automated compliance tracking.
📍 Book a Demo
📧 hello@bprhub.com
Common Documentation Requirements for Food and Drug Administration Certificate
Product labeling compliance forms the foundation of successful food and drug administration certificate applications. Labels must include complete ingredient lists, nutritional information, allergen declarations, and manufacturing facility information according to FDA labeling regulations.
Manufacturing process documentation demonstrates your facility's ability to produce safe, consistent products. Include detailed process flow charts, critical control points, hazard analysis documentation, and quality assurance procedures. Foreign regulators scrutinize these documents to ensure your products meet their safety standards.
Certificate applications require current facility inspection reports, cGMP compliance documentation, and evidence of proper sanitation procedures. Maintain organized records of all inspections, corrective actions, and ongoing compliance monitoring to support your application.
Ingredient sourcing documentation proves the safety and legality of all product components. Provide supplier certifications, country of origin information, and any relevant testing results for imported ingredients. Foreign authorities often require detailed supply chain transparency before approving imports.
Navigating International Market-Specific Export Certification Requirements
European Union markets demand additional certifications beyond standard export certification, including compliance with EU Novel Food Regulations for products containing innovative ingredients. Manufacturers must provide detailed safety assessments and scientific studies supporting ingredient safety for EU market entry.
Asian markets, particularly Japan and South Korea, require country-specific health certificates alongside FDA documentation. Japan's Ministry of Health, Labour, and Welfare often requests radiation testing certificates for food products, while South Korea emphasizes Korean language labeling compliance documentation.
Canada's Food and Drug Act requires additional documentation for processed foods, including detailed manufacturing process descriptions and quality control procedures. Canadian authorities frequently request facility inspection reports and evidence of HACCP implementation beyond FDA requirements.
Middle Eastern markets present unique challenges, with many countries requiring Halal certification alongside FDA documentation. Coordinate with recognized Halal certification bodies early in your export planning to avoid documentation conflicts or delays.
Read more on - 4 Types of FDA Inspections: What You Need to Know
Maintaining Compliance for Ongoing FDA Export Certificate Validity
Annual facility registration renewal ensures continuous eligibility for FDA export certificate issuance. Submit renewal applications 60-90 days before expiration to avoid lapses that could disrupt export operations. Late renewals trigger additional fees and potential processing delays.
Regular facility inspections maintain compliance with FDA standards required for certificate validity. Prepare for unannounced inspections by maintaining current documentation, training records, and corrective action logs. Inspection failures can suspend certificate issuance indefinitely.
Product formulation changes require notification to the FDA and may necessitate new certificate applications. Major ingredient modifications, processing changes, or facility relocations trigger mandatory reapplication processes. Maintain detailed change control procedures to ensure compliance and continuity. Monitor evolving FDA regulations affecting your product categories through official FDA communications and industry associations. Regulatory changes can impact certificate requirements, processing times, and documentation standards without advance notice.
How BPR Hub Simplifies FDA Certification and Export Certification Management
BPR Hub's Unified Compliance Framework centralizes FDA certification documentation management, reducing preparation time from weeks to days through automated document generation and real-time compliance monitoring. Food manufacturers access pre-configured templates aligned with FDA requirements, eliminating manual documentation errors that delay certificate processing.
The platform's audit management system tracks facility inspection readiness, maintains current cGMP compliance documentation, and provides automated alerts for registration renewals. Manufacturers stay ahead of compliance deadlines while reducing the administrative burden on internal teams.
Real-time regulatory monitoring keeps manufacturers informed of FDA policy changes affecting export certification requirements. Automated notifications ensure teams respond promptly to regulatory updates, maintaining continuous compliance without manual policy tracking.
BPR Hub's document control system maintains version management for all certification documentation, providing auditors and FDA inspectors with immediate access to current procedures and historical records. Centralized storage eliminates the risk of submitting outdated documentation during certificate applications.
Accelerate your international expansion, get FDA-ready documentation with BPR Hub.
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ FDA export certificates require facility registration, comprehensive product documentation, and adherence to current Good Manufacturing Practices before international food trade
→ FDA certification cost ranges from $175-$2,340, depending on processing speed and facility size, with small business discounts available for qualifying manufacturers
→ Processing timelines span 20-60 business days for standard applications, while expedited services deliver certificates within 5-10 business days for urgent shipments
→ International markets impose additional requirements beyond FDA documentation, including EU Novel Food compliance, Halal certification, and country-specific health certificates
→ Maintaining compliance requires annual facility registration renewal, regular inspections, and prompt notification of product formulation changes to prevent certificate suspension
→ Automated compliance management platforms reduce export certification preparation time by 60% through centralized documentation control and real-time regulatory monitoring
FAQ
Q. How much does the FDA charge for an export certificate?
FDA charges between $175-$585 for standard FDA export certificate processing, with expedited services ranging up to $2,340 for urgent requests. Small businesses qualifying for reduced fee structures pay approximately 50% less than standard rates. Additional costs include annual facility registration fees and potential consultation expenses for complex applications. Fee structures vary by product type, facility size, and processing timeline requirements, making advance budgeting essential for international expansion planning.
Q. What is the export certification of the FDA?
Export certification from the FDA serves as official documentation confirming a food product's regulatory status within the United States for international trade purposes. The certificate provides foreign governments with detailed information about manufacturing facility compliance, product formulation safety, and regulatory history. The FDA issues these certificates to facilitate product exports when requested by foreign customers or regulatory authorities. Certificates remain valid for specific time periods and require renewal based on facility registration status and ongoing compliance maintenance.
Q. Do I need a license to export food from the USA?
Food manufacturers do not require separate export licenses but must maintain current FDA facility registration and comply with applicable food safety standards. FDA export certificates serve as proof of regulatory compliance rather than licensing documents. However, certain specialty products may require additional permits from other agencies, such as the USDA for meat products or the TTB for alcoholic beverages. Manufacturers must also comply with destination country import requirements, which may include additional certifications beyond FDA documentation.
Q. How to get a certificate of export?
Obtaining FDA export certificates begins with facility registration through the FDA's online portal using Form FDA 2541. Manufacturers prepare comprehensive product documentation, including ingredient lists, manufacturing processes, and quality control procedures aligned with FDA requirements. Submit applications through the FDA electronic system with all supporting documents and applicable fees. Processing requires 20-60 business days for standard applications, with expedited options available for urgent shipments requiring faster processing timelines.
Q. How to become an approved exporter?
Approved exporter status requires maintaining current FDA facility registration, demonstrating cGMP compliance, and establishing documented quality management systems. Manufacturers must pass FDA facility inspections, maintain proper sanitation procedures, and implement effective hazard analysis protocols. Continuous compliance monitoring ensures ongoing eligibility for export certification issuance. Additional requirements may apply based on specific product categories, destination countries, and international trade agreements affecting your manufacturing operations.
Q. How long does FDA export certificate processing take?
Standard FDA export certificate processing requires 20-60 business days, depending on application complexity and FDA workload. Expedited processing options deliver certificates within 5-10 business days for additional fees ranging $500-$1,200. Rush processing provides certificates within 2-3 business days at premium pricing up to $2,340 for critical shipments. Processing delays commonly result from incomplete documentation, facility compliance issues, or high application volumes during peak export seasons, requiring planning for international shipments.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

