When manufacturers face the challenge of choosing between CMMI and ISO 9001 standards, the decision becomes critical for operational excellence and competitive advantage. Both frameworks aim to improve organizational processes, yet they approach quality management through distinctly different pathways. Understanding the difference between CMMI and ISO standards empowers manufacturers to make informed decisions that align with their growth objectives and compliance requirements.
This comprehensive guide explores how these two internationally recognized standards can transform your manufacturing operations, streamline compliance workflows, and accelerate market entry, particularly when supported by BPRHub, that centralize multi-standard management.
Understanding CMMI, Process Improvement Through Maturity Levels
CMMI (Capability Maturity Model Integration) represents a comprehensive framework designed to enhance organizational processes across multiple domains. Originally developed by the Software Engineering Institute at Carnegie Mellon University, CMMI has evolved beyond software development to encompass manufacturing, services, and acquisition processes.
What are the Core Components of the CMMI Framework?
CMMI operates through a structured approach that emphasizes continuous improvement:
Maturity Levels: Five distinct levels ranging from Initial (Level 1) to Optimizing (Level 5)
Process Areas: 22 specific domains covering everything from requirements management to organizational performance
Capability Levels: Six levels (0-5) that measure individual process area performance
Appraisal Methods: Formal evaluation processes conducted by certified lead appraisers
What are the Five Levels of CMMI?
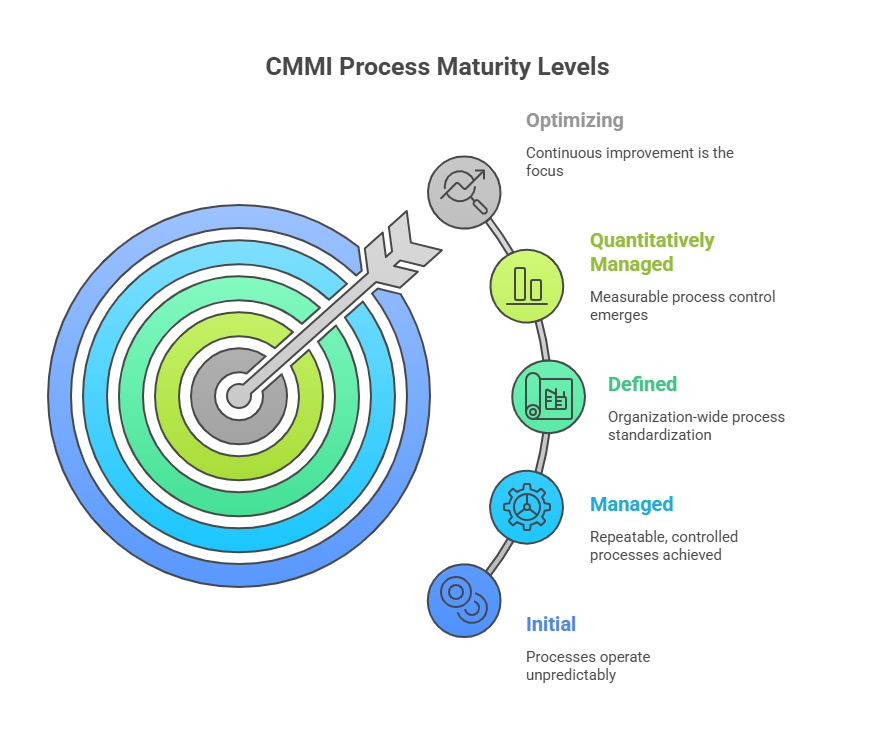
Level 1 - Initial: Processes operate unpredictably and reactively
- Organizations function in crisis mode with ad-hoc approaches
- Success depends on individual heroics rather than systematic processes
- Limited process documentation exists across the organization
Level 2 - Managed: Projects achieve repeatable, controlled processes
- Basic project management practices become established
- Requirements and work products receive systematic control
- Process discipline helps ensure existing practices retain their effectiveness
Level 3 - Defined: Organization-wide process standardization takes hold
- Processes become well-characterized and thoroughly understood
- Standards, procedures, tools, and methods receive comprehensive documentation
- Projects tailor organizational standard processes to meet specific needs
Level 4 - Quantitatively Managed: Measurable process control emerges
- Quantitative objectives for quality and performance become established
- Process performance is controlled through statistical techniques
- Predictable process performance is achieved consistently
Level 5 - Optimizing: Continuous improvement becomes the focus
- Processes receive continuous improvement through incremental innovations
- Quantitative process improvement objectives become established
- Organizations pilot innovative improvements to optimize processes
ISO 9001: Quality Management System Excellence
ISO 9001 stands as the world's most widely recognized quality management standard, providing a framework for organizations to demonstrate their ability to consistently provide products and services that meet customer and regulatory requirements.
What are the Fundamental Principles of ISO 9001
The standard builds upon seven quality management principles:
Customer Focus: Understanding and meeting customer needs drives all activities
Leadership: Leaders establish unity of purpose and direction throughout the organization
Engagement of People: Competent, empowered, and engaged people enhance organizational capability
Process Approach: Activities become managed as interrelated processes
Improvement: Successful organizations maintain focus on continual improvement
Evidence-based Decision Making: Decisions rely on analysis of data and information
Relationship Management: Organizations manage relationships with relevant interested parties
ISO 9001 Structure and Requirements
The current ISO 9001:2015 version follows the High-Level Structure (HLS) common to all ISO management system standards:
Clause 1 - Context of the Organization
- Understanding organizational context and stakeholder needs becomes essential
- Defining QMS scope and processes requires careful consideration
Clause 2 - Leadership
- Top management commitment and accountability drive success
- Quality policy establishment and communication ensure alignment
Clause 3 - Planning
- Risk-based thinking integration becomes fundamental
- Quality objectives and planning help achieve desired outcomes
Clause 4 - Support
- Resource management includes people, infrastructure, and environment
- Competence, awareness, and communication requirements support operations
Clause 5 - Operation
- Operational planning and control ensure consistent delivery
- Product and service requirements management maintains quality
- Design, development, and production control enable excellence
Clause 6 - Performance Evaluation
- Monitoring, measurement, analysis, and evaluation provide insights
- Internal audit and management review processes ensure effectiveness
Clause 7 - Improvement
- Nonconformity correction and corrective action prevent recurrence
- Continual improvement commitment drives organizational growth
Key Similarities: Where CMMI and ISO 9001 Converge
Despite their different approaches, CMMI and ISO 9001 share fundamental commonalities that make them complementary rather than competing frameworks.
Process-Oriented Methodology
Both standards emphasize the importance of well-defined, documented processes:
- Systematic Approach: Each framework requires organizations to identify, document, and manage key processes
- Process Improvement: Continuous enhancement forms the foundation of both methodologies
- Measurement and Analysis: Data-driven decision making receives emphasis in both standards
- Risk Management: Proactive identification and mitigation of risks becomes integral to both approaches
Customer-Centric Focus
Manufacturing organizations benefit from both standards' emphasis on customer satisfaction:
- Requirements Management: A Clear understanding and documentation of customer needs drives success
- Feedback Integration: Systematic collection and analysis of customer feedback enables improvement
- Continuous Enhancement: Using customer insights to drive improvement initiatives creates value
- Quality Delivery: Ensuring consistent delivery of products and services meeting specifications
International Recognition and Credibility
Both frameworks provide global credibility for manufacturing operations:
- Market Access: International recognition facilitates entry into new markets
- Supply Chain Integration: Major OEMs often require suppliers to demonstrate compliance
- Competitive Advantage: Standards implementation differentiates organizations in competitive markets
- Regulatory Alignment: Both standards support compliance with various regulatory requirements
Confused between CMMI and ISO 9001? Let BPRHub simplify your decision. Book a demo today.
📍 Book a Demo
📧 hello@bprhub.com
What are the Critical Differences Between ISO 9001 and CMMI
Understanding the difference between CMMI and ISO standards requires examining their fundamental approaches, scope, and implementation methodologies.
Scope and Application Differences
CMMI Scope:
- Comprehensive framework covering multiple organizational functions
- Addresses software development, systems engineering, integrated product development, and supplier sourcing
- Provides specific guidance for process improvement initiatives
- Focuses on organizational capability enhancement across domains
ISO 9001 Scope:
- Quality management system requirements apply to any organization
- Emphasizes customer satisfaction and regulatory compliance
- A generic standard adaptable to various industries and organization sizes
- Process-based approach with risk-based thinking integration
Implementation Timeline and Complexity
CMMI Implementation
- Typically requires 18-36 months for significant maturity level advancement
- Demands substantial organizational culture change and commitment
- Requires dedicated process improvement teams and resources
- Investment in training and infrastructure often exceeds $500,000 for medium-sized organizations
ISO 9001 Implementation
- Generally achievable within 6-18 months for initial audit readiness
- Can leverage existing quality management practices as a foundation
- Requires documented QMS but allows flexibility in approach
- Implementation costs typically range from $50,000-$200,000, depending on organization size
Manufacturing Industry Applications: Real-World Implementation
Aerospace and Defense Manufacturing
The aerospace sector demonstrates how both standards complement each other:
CMMI Benefits
- AS9100 alignment through enhanced process maturity
- Improved supplier qualification processes and management
- Enhanced project management capabilities across programs
- Risk reduction in complex manufacturing programs
ISO 9001 Benefits
- Customer requirement satisfaction (often mandatory for contracts)
- International market access facilitation and credibility
- Regulatory compliance demonstration to the authorities
- Supply chain integration support and vendor management
Pharmaceutical and Medical Device Manufacturing
Healthcare manufacturing presents unique compliance challenges:
CMMI Applications
- Process validation and control enhancement across operations
- Supplier quality management improvement and oversight
- Technology transfer optimization between sites
- Risk management framework development and implementation
ISO 9001 Applications
- FDA 21 CFR Part 820 alignment and compliance support
- Quality management system foundation establishment
- CAPA (Corrective and Preventive Action) process enhancement
- Documentation control and management systems
Automotive Manufacturing
The automotive industry leverages both standards for operational excellence:
CMMI Value Proposition
- APQP (Advanced Product Quality Planning) process improvement
- Supplier development program enhancement and management
- Lean manufacturing integration and optimization
- Continuous improvement culture development
ISO 9001 Value Proposition
- IATF 16949 foundation establishment and maintenance
- Customer-specific requirement management systems
- Global supply chain integration and coordination
- Quality cost reduction initiatives and programs
Before BPRHub
Manufacturing organizations implementing both CMMI and ISO 9001 face significant operational challenges
- Duplicate Documentation: Teams maintain separate document repositories for each standard
- Fragmented Processes: Compliance activities operate in silos without integration
- Audit Preparation Chaos: CMMI audit and ISO 9001 assessments require separate preparation efforts
- Resource Inefficiency: Multiple teams work on overlapping compliance requirements
- Risk Exposure: Gaps between standards create potential non-compliance issues
With BPRHub
BPRHub's compliance management platform transforms how manufacturers approach CMMI and ISO 9001 implementation through centralized capabilities:
Centralized Documentation Management: Single repository consolidates all process documentation across standards
Integrated Audit Management: Management with corrective action tracking across both standards
Process Optimization Across Standards: BPRHub enables manufacturers to leverage synergies between both frameworks
Risk Management Integration: The unified risk register covers both standard requirements comprehensively
Implementation Strategy for Choosing Between CMMI and ISO 9001
Decision Framework for Manufacturing Organizations
Choose CMMI When:
- The organization seeks comprehensive process improvement across multiple domains
- Industry demands demonstrated process capability (aerospace, defense, software)
- Long-term organizational transformation and culture change become the priority
- Resources become available for substantial investment in process improvement
Choose ISO 9001 When:
- Customer or regulatory requirements mandate quality management system standards
- The organization needs an internationally recognized quality credential quickly
- Limited resources require a focused quality management approach
- Rapid standards achievement becomes desired for market access
Implement Both When:
- The organization operates in highly regulated industries requiring both process maturity and quality system compliance
- Competitive advantage requires demonstration of both process capability and quality management excellence
- Resources permit a comprehensive approach to operational excellence
- Supply chain requirements include both standards for vendor qualification
What is the Sequential Implementation Approach
Phase 1: Foundation Building (Months 1-6)
- Establish a basic quality management system per ISO 9001 requirements
- Document core processes and procedures across the organization
- Implement measurement and monitoring systems for key processes
- Conduct a comprehensive gap analysis for both standards
Phase 2: Process Maturity Development (Months 7-18)
- Begin CMMI implementation focusing on Level 2 requirements
- Integrate ISO 9001 processes with CMMI process areas
- Establish quantitative management practices across operations
- Develop organizational process assets and documentation
Phase 3: Optimization and Integration (Months 19-36)
- Achieve CMMI Level 3 organizational standardization
- Pursue ISO 9001 standards assessment and compliance
- Implement continuous improvement programs organization-wide
- Establish a metrics-driven management culture
How BPRHub Helps with ISO 9001 and CMMI Implementation
Unified Compliance Framework
BPRHub's comprehensive platform addresses the difference between CMMI and ISO standards by providing:
- Centralized Management: Single platform manages both CMMI and ISO 9001 requirements
- Process Integration: Automated mapping between standards eliminates duplication
- Real-time Monitoring: Continuous compliance tracking across all standards
- Automated Reporting: Executive dashboards provide visibility into compliance status
Streamlined Audit Readiness
Transform your audit preparation with BPRHub's integrated approach:
- Evidence Collection: Automated gathering of audit evidence for both standards
- Gap Analysis: Real-time identification of compliance gaps and remediation actions
- Workflow Automation: Streamlined processes reduce manual effort and errors
- Collaborative Platform: Teams collaborate effectively across both standards
- Operational Excellence Enablement
BPRHub empowers manufacturers to achieve operational excellence through:
- Process Optimization: Identify and eliminate redundancies across standards
- Resource Efficiency: Reduce costs through integrated compliance management
- Risk Mitigation: Proactive identification and management of compliance risks
- Scalable Growth: Standards become enablers rather than barriers to growth
Streamline compliance and accelerate growth. Let BPRHub manage your CMMI and ISO 9001.
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ CMMI focuses on process improvement through maturity levels, while ISO 9001 emphasizes quality management system requirements, both drive operational excellence through complementary approaches
→ The difference between CMMI and ISO standards lies in scope and methodology: CMMI provides comprehensive organizational capability enhancement while ISO 9001 ensures quality management system compliance
→ Manufacturing organizations achieve maximum benefit when implementing both standards sequentially, starting with ISO 9001 for foundational quality management, then advancing to CMMI for process maturity
→ CMMI audit processes differ significantly from ISO 9001 assessments, requiring formal appraisal methods versus third-party audit approaches
→ BPRHub's Unified Compliance Framework eliminates duplicate efforts by centralizing documentation, automating compliance monitoring, and providing real-time visibility across both standards
→ Strategic implementation of both standards creates a competitive advantage through demonstrated process capability, international quality recognition, and enhanced customer confidence in manufacturing operations
FAQ
Q. What is the difference between CMMI and ISO 9001?
CMMI focuses on process improvement and organizational capability development through five maturity levels, while ISO 9001 establishes quality management system requirements for consistent product and service delivery. CMMI emphasizes incremental process enhancement across multiple domains, whereas ISO 9001 provides a framework for meeting customer and regulatory requirements through systematic quality management approaches.
Q. What is the difference between CMMI and ISO based on scope, approach, and implementation?
CMMI covers comprehensive organizational processes, including development, acquisition, and services, while ISO 9001 focuses specifically on quality management systems. Approach: CMMI uses maturity-based progression through defined levels, while ISO 9001 requires full compliance with all applicable clauses. Implementation: CMMI typically requires 18-36 months for maturity advancement with substantial cultural change, while ISO 9001 can be implemented within 6-18 months with existing quality practices as a foundation.
Q. What are the five levels of CMMI?
The five levels of CMMI are
Level 1 (Initial) - unpredictable and reactive processes
Level 2 (Managed) - repeatable project-level processes
Level 3 (Defined) - organization-wide process standardization
Level 4 (Quantitatively Managed) - measurable process control using statistical techniques
Level 5 (Optimizing) - continuous process improvement through innovation.
Each level builds upon previous achievements, creating systematic organizational capability enhancement.
Q. What is the difference between ISO 9001 and Agile?
ISO 9001 represents quality management standards requiring documented processes, risk management, and continuous improvement, while Agile represents project management methodologies emphasizing iterative development and flexibility. ISO 9001 can accommodate Agile methodologies within its framework—many organizations successfully implement Agile practices while maintaining ISO 9001 compliance through documented procedures that support iterative approaches and customer collaboration.
Q. What is the primary purpose of ISO 9001?
The primary purpose of ISO 9001 involves providing organizations with a framework for establishing, implementing, and maintaining a quality management system that consistently delivers products and services meeting customer requirements and applicable regulations. The standard aims to enhance customer satisfaction through effective system application, continual improvement processes, and assurance of conformity to customer and regulatory requirements while facilitating international trade and market access.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

