Manufacturing professionals often encounter confusion when navigating between GMP and cGMP requirements, leading to compliance gaps that can result in costly regulatory violations. According to the FDA's Inspection Dashboard, pharmaceutical manufacturing facilities continue to receive significant numbers of warning letters for current good manufacturing practices violations that could have been prevented with a proper understanding of regulatory expectations.
The difference between cGMP and GMP lies not just in terminology but in fundamental approaches to quality management that directly impact operational efficiency and regulatory compliance. Understanding these distinctions empowers manufacturers to implement the right standards for their specific industry requirements while avoiding over-compliance costs.
This comprehensive guide clarifies the cGMP vs GMP debate, providing actionable insights that transform regulatory complexity into competitive advantage for modern manufacturers.
What is GMP Meaning and Why Does It Matter for Manufacturers
GMP meaning encompasses the fundamental quality principles that ensure manufactured products meet safety, efficacy, and quality requirements consistently. These baseline standards establish minimum requirements for personnel, facilities, equipment, and processes across various manufacturing industries.
Good Manufacturing Practices serve as the foundation for quality systems worldwide, providing manufacturers with systematic approaches to product quality that protect consumers while supporting business objectives. The principles transcend industry boundaries, offering scalable frameworks that adapt to different manufacturing environments.
Core Components of Good Manufacturing Practices Standards
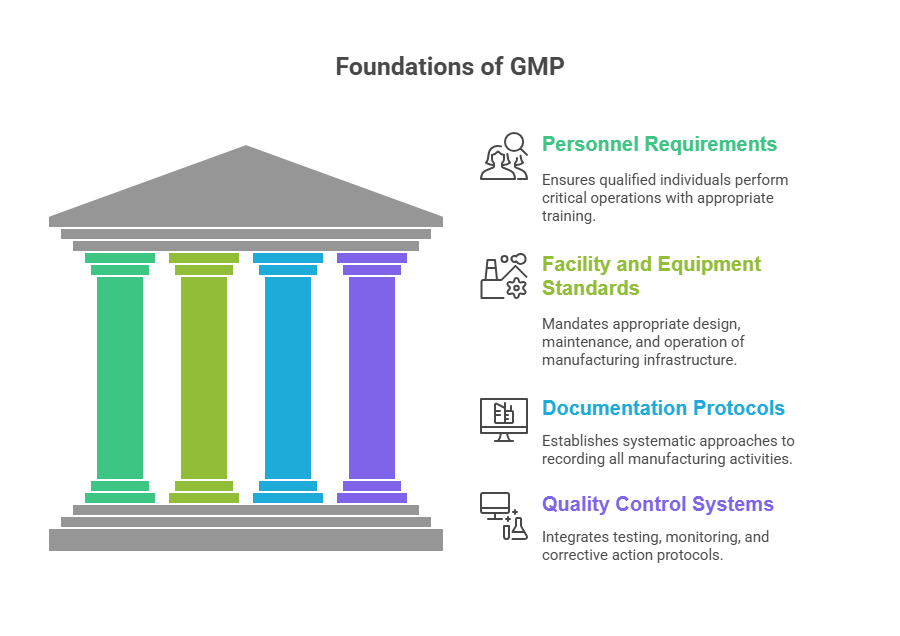
Good manufacturing practices rest on four essential pillars that create comprehensive quality frameworks. Personnel requirements ensure that qualified individuals perform critical operations with appropriate training and oversight. These requirements establish competency standards that link individual performance to product quality outcomes.
Facility and equipment standards mandate appropriate design, maintenance, and operation of manufacturing infrastructure. These requirements ensure that physical environments support product quality while preventing contamination and cross-contamination that could compromise safety.
Documentation protocols establish systematic approaches to recording all manufacturing activities, decisions, and outcomes. Comprehensive documentation provides evidence of consistent implementation while supporting continuous improvement initiatives that drive operational excellence.
Quality control systems integrate testing, monitoring, and corrective action protocols that verify product conformity before release. These systems provide multiple verification points that catch potential issues before they reach customers.
Streamline your GMP to cGMP transition with BPRHub. Explore how our unified compliance platform simplifies regulatory excellence.
📍 Book a Demo
📧 hello@bprhub.com
GMP Regulations Across Different Industries
GMP regulations vary significantly across industries while maintaining consistent core principles. The pharmaceutical industry implements the most stringent requirements due to direct patient safety implications, requiring comprehensive validation and documentation at every process step as mandated by 21 CFR Parts 210 and 211.
Food and beverage manufacturing applies GMP principles through HACCP systems that focus on biological, chemical, and physical hazards. These applications emphasize prevention strategies that eliminate contamination risks throughout production processes.
Medical device production incorporates GMP requirements through ISO 13485 and FDA Quality System Regulation (21 CFR Part 820) frameworks. These applications balance product safety requirements with innovation needs that drive medical technology advancement.
Current Good Manufacturing Practices Explained
cGMP meaning extends beyond traditional GMP by incorporating contemporary regulatory expectations, technological capabilities, and risk-based approaches to quality management. The "current" designation emphasizes that these practices evolve with advancing technology and regulatory science.
Current good manufacturing practices represent dynamic standards that adapt to changing manufacturing environments while maintaining rigorous quality requirements. This flexibility enables manufacturers to leverage technological advances while ensuring continued regulatory compliance.
What Makes Current Good Manufacturing Practices "Current"
Current good manufacturing practices distinguish themselves through integration of modern technologies that enhance quality capabilities beyond traditional manual systems. Real-time monitoring, automated data capture, and predictive analytics transform reactive quality approaches into proactive prevention strategies.
Updated regulatory expectations reflect advances in manufacturing science, risk assessment methodologies, and quality system thinking. These expectations require manufacturers to demonstrate systematic understanding of their processes while implementing controls based on scientific rationale rather than prescriptive compliance.
Modern quality systems approach emphasizes process understanding, continuous monitoring, and data-driven decision making. This approach enables manufacturers to optimize operations while maintaining quality through intelligent system design rather than extensive testing and inspection.
cGMP Manufacturing Requirements and Implementation
cGMP manufacturing requirements mandate sophisticated quality systems that leverage technology for enhanced process control and data integrity. Real-time monitoring systems provide continuous oversight of critical parameters while generating comprehensive data records that support regulatory submissions.
Risk-based approaches enable manufacturers to focus resources on areas with greatest potential impact on product quality. These approaches require systematic risk assessment, control implementation, and effectiveness monitoring that demonstrate scientific understanding of manufacturing processes.
Continuous improvement protocols ensure that quality systems evolve with changing conditions, regulatory expectations, and technological capabilities. These protocols create learning organizations that systematically enhance their quality capabilities over time.
cGMP vs GMP, Side-by-Side Comparison Analysis
cGMP vs GMP comparison reveals fundamental differences in scope, implementation approaches, and regulatory expectations. While GMP provides foundational quality principles, cGMP demands contemporary implementation that leverages current technology and regulatory science.
The distinction impacts resource allocation, technology investment, and organizational capabilities required for successful implementation. Understanding these differences enables informed decisions about compliance strategies that balance requirements with business objectives.
Key Differences in GMP vs cGMP Standards
GMP cGMP difference centers on static versus dynamic compliance approaches. Traditional GMP relies on established procedures and manual verification methods that provide consistent but potentially limited quality oversight. cGMP demands adaptive systems that respond to changing conditions while maintaining control.
Documentation requirements differ significantly between approaches. GMP emphasizes paper-based records and manual signature processes, while cGMP leverages electronic systems with enhanced data integrity, automated workflow, and real-time access capabilities.
Quality system sophistication varies dramatically. GMP implementations often rely on basic testing and inspection protocols, whereas cGMP integrates process analytical technology, statistical process control, and predictive quality management that prevent issues before their occurrence.
Cost Implications of GMP vs cGMP Implementation
Initial investment requirements for cGMP implementation typically exceed traditional GMP due to technology infrastructure, system validation, and personnel training needs. However, these investments generate significant long-term operational benefits through reduced quality failures, faster batch release, and improved regulatory relationships.
Long-term operational benefits include reduced inspection frequency, accelerated product approvals, and enhanced market access opportunities. cGMP compliance demonstrates regulatory leadership that supports business expansion into new markets and product categories.
Return on investment considerations must account for risk reduction, operational efficiency, and competitive advantages beyond direct cost savings. cGMP implementation often enables premium pricing, preferred supplier status, and strategic partnership opportunities that justify initial investments.

FDA, GMP and cGMP Guidelines
FDA GMP guidelines establish minimum quality standards for products under the Food and Drug Administration jurisdiction. These guidelines provide specific requirements for pharmaceutical, medical device, and food manufacturing that ensure consumer safety while supporting industry innovation.
The Food and Drug Administration maintains comprehensive inspection programs that verify compliance with applicable GMP or cGMP requirements. Understanding regulatory expectations enables manufacturers to prepare effectively for inspections while maintaining continuous compliance.
Food and Drug Administration Oversight Role
Food and Drug Administration oversight includes comprehensive inspection protocols that evaluate quality system effectiveness across all manufacturing operations. Inspectors assess documentation, interview personnel, and observe operations to verify systematic implementation of quality requirements.
Enforcement mechanisms range from warning letters and consent decrees to product seizures and facility shutdowns. Understanding these mechanisms enables manufacturers to prioritize compliance investments while avoiding potential regulatory actions that could disrupt operations.
Compliance expectations continue evolving with advances in manufacturing science, technology capabilities, and regulatory frameworks. Staying current with these expectations requires systematic monitoring of guidance documents, industry communications, and regulatory science publications.
cGMP Compliance Requirements for Pharmaceutical Companies
Pharmaceutical companies face the most stringent cGMP requirements due to direct patient safety implications. These requirements mandate comprehensive validation of manufacturing processes, analytical methods, and quality systems that demonstrate consistent product quality.
Documentation standards require electronic records with appropriate data integrity controls, audit trails, and access management. These standards ensure that all quality decisions are based on accurate, complete, and traceable information.
Quality assurance protocols must demonstrate systematic oversight of all manufacturing activities through independent review, testing, and approval processes. These protocols provide multiple verification points that ensure product quality before release to patients.
GMP Manufacturing vs cGMP Manufacturing
GMP manufacturing applications vary significantly across industries while maintaining consistent quality principles. Understanding industry-specific requirements enables manufacturers to implement appropriate quality systems without over-engineering compliance solutions.
The pharmaceutical industry leads cGMP implementation due to regulatory requirements and patient safety considerations. Other industries are increasingly adopting cGMP principles to achieve competitive advantages through superior quality capabilities.
Pharmaceutical Industry Implementation Standards
Pharmaceutical industry implementations require comprehensive process validation that demonstrates consistent product quality under normal operating conditions. Validation protocols must address all critical aspects, including equipment, facilities, utilities, and personnel qualifications.
Drug development protocols integrate quality considerations from early research through commercial manufacturing. This integration ensures that quality systems support product development while meeting regulatory expectations for market approval.
Production facility requirements mandate appropriate design, environmental controls, and segregation that prevent contamination and mix-ups. These requirements create controlled environments that support consistent product quality throughout manufacturing operations.
cGMP Pharmaceutical Standards and Best Practices
cGMP pharmaceutical standards emphasize process understanding, continuous monitoring, and data-driven decision making. Advanced manufacturing technologies enable real-time process control that ensures consistent product quality while optimizing manufacturing efficiency.
Data integrity requirements mandate comprehensive controls over electronic records, including access management, audit trails, and backup procedures. These requirements ensure that quality decisions are based on accurate, complete, and reliable information.
Supply chain management requirements extend quality oversight to raw material suppliers, contract manufacturers, and distribution partners. This comprehensive approach ensures product quality throughout the entire product lifecycle.
GMP Compliance vs cGMP Compliance
GMP compliance strategies focus on meeting minimum regulatory requirements through documented procedures and training programs. These strategies provide baseline quality capabilities that satisfy regulatory expectations while minimizing compliance costs.
cGMP compliance demands sophisticated quality systems that leverage technology for enhanced process control and continuous improvement. These strategies require significant investment but generate superior quality capabilities that support business growth.
Essential GMP Requirements for Manufacturers
GMP requirements establish minimum standards for personnel qualifications, facility design, equipment maintenance, and documentation practices. These requirements create foundational quality capabilities that prevent basic quality failures while satisfying regulatory expectations.
Basic quality systems include documented procedures for all critical operations, personnel training programs, and basic testing protocols. These systems provide systematic approaches to quality management that ensure consistent implementation across manufacturing operations.
Standard operating procedures must address all aspects of manufacturing operations, including material handling, production processes, quality control testing, and product release. These procedures provide detailed guidance that ensures consistent execution regardless of personnel changes.
Advanced cGMP Requirements and Standards
cGMP requirements mandate sophisticated quality systems that integrate process analytical technology, real-time monitoring, and predictive analytics. These requirements enable proactive quality management that prevents issues before they impact product quality.
Technology integration mandates include electronic batch records, automated data capture, and real-time process monitoring systems. These technologies provide comprehensive oversight capabilities that exceed traditional manual verification methods.
Enhanced data management requirements ensure that all quality-related information is accurate, complete, and readily accessible for decision making. Advanced systems provide analytical capabilities that identify trends and patterns that support continuous improvement.
GMP Training vs cGMP Training
GMP training provides foundational knowledge about quality principles, regulatory requirements, and basic implementation practices. These programs ensure that personnel understand their roles in maintaining product quality while meeting regulatory expectations.
Advanced cGMP training addresses sophisticated quality concepts, including process validation, risk assessment, and technology integration. These programs develop the expertise needed to implement and maintain state-of-the-art quality systems.
Building Competency in Good Manufacturing Practices Guidelines
GMP guidelines training focuses on regulatory requirements, documentation practices, and basic quality procedures. Core curriculum components include personnel hygiene, contamination prevention, and basic testing protocols that ensure consistent product quality.
Skill development priorities emphasize practical application of quality principles through hands-on exercises and case studies. These approaches ensure that personnel can apply training content to real manufacturing situations while maintaining regulatory compliance.
Assessment methodologies verify understanding through written examinations, practical demonstrations, and ongoing performance monitoring. These assessments ensure that personnel maintain competency over time while adapting to changing requirements.
Advanced cGMP Certification Programs
cGMP certification programs provide specialized training in advanced quality concepts, including process validation, risk-based approaches, and technology integration. These programs develop the expertise needed to lead quality system implementation and improvement initiatives.
Specialized training modules address industry-specific requirements for pharmaceutical, medical device, and biotechnology manufacturing. These modules provide targeted knowledge that addresses unique challenges and opportunities within specific manufacturing sectors.
Continuing education needs to ensure that quality professionals stay current with evolving regulatory expectations, technology capabilities, and industry best practices. Ongoing education maintains expertise while supporting career development in quality management roles.
Transitioning from GMP Standards to cGMP Standards
GMP standards provide the foundation for advancing to cGMP implementation through systematic capability development. Successful transitions require careful planning, resource allocation, and change management that minimizes operational disruption while building quality capabilities.
The transition process involves a comprehensive assessment of current capabilities, identification of gaps, and systematic implementation of enhanced quality systems. This structured approach ensures successful cGMP implementation while maintaining continuous operations.
Assessment and Gap Analysis Process
Current state evaluation identifies existing quality capabilities, documentation systems, and technology infrastructure. This assessment provides a baseline understanding of organizational readiness for cGMP implementation while highlighting areas requiring development.
Compliance gap identification compares current capabilities against cGMP requirements to prioritize improvement initiatives. This analysis ensures that resources focus on areas with the greatest impact on regulatory compliance and operational performance.
Priority setting methodology balances regulatory requirements, business objectives, and resource constraints to create realistic implementation timelines. This systematic approach ensures sustainable progress while maintaining operational effectiveness.
System Upgrades and Technology Integration
Infrastructure modernization includes facility improvements, equipment upgrades, and utility system enhancements that support cGMP requirements. These improvements create environments capable of supporting advanced quality systems while maintaining operational efficiency.
Software implementation focuses on electronic quality management systems that automate workflows, capture data electronically, and provide real-time visibility into quality metrics. Modern platforms like BPRHub's Unified Compliance Framework integrate multiple standards within a single interfaces that eliminate duplicate workflows.
Equipment validation ensures that all manufacturing and testing equipment operates within specified parameters while maintaining appropriate documentation. Validation activities demonstrate that equipment consistently produces expected results under normal operating conditions.
Staff Development and Change Management
Training program design addresses both technical skills and change management needs that support cGMP implementation. Comprehensive programs ensure that personnel understand new requirements while developing capabilities needed for successful implementation.
Cultural transformation initiatives align organizational values with cGMP principles, including continuous improvement, data-driven decision making, and proactive quality management. These initiatives create environments that support sustained cGMP compliance.
Performance monitoring systems track implementation progress while identifying areas requiring additional support or resources. These systems ensure that cGMP implementation achieves intended outcomes while maintaining operational performance.
How BPRHub Helps with cGMP Implementation
BPRHub transforms the complexity of cGMP vs GMP implementation challenges into streamlined competitive advantages through our comprehensive Quality, Compliance, and Governance platform. Our Unified Compliance Framework centralizes current good manufacturing practices management within intelligent systems that eliminate duplicate workflows while ensuring continuous audit readiness.
Our platform addresses the full spectrum of cGMP requirements through integrated modules that support document control, risk management, training management, and audit preparation. The system automatically maintains compliance with evolving regulatory expectations while providing real-time visibility into quality metrics across all manufacturing operations.
BPRHub's advanced technology integration capabilities enable seamless transition from traditional GMP to cGMP implementation through automated workflow routing, electronic signature capture, and comprehensive audit trails. Our platform supports over 30 regulatory standards simultaneously, enabling manufacturers to achieve comprehensive compliance while reducing administrative overhead.
Ready to turn compliance into a competitive edge? Discover how BPRHub helps manufacturers achieve seamless cGMP implementation.
📍 Book a Demo
📧 hello@bprhub.com
Key Takeaways
→ cGMP vs GMP represents evolution from basic quality requirements to sophisticated systems that leverage current technology and regulatory science for enhanced manufacturing control
→ Current good manufacturing practices demand dynamic compliance approaches that adapt to changing conditions while maintaining rigorous quality standards across all operations
→ GMP compliance provides foundational quality capabilities while cGMP compliance enables advanced manufacturing that supports premium market positioning and regulatory leadership
→ FDA GMP requirements establish minimum standards, while cGMP implementation demonstrates regulatory leadership that accelerates approvals and market access opportunities
→ GMP training builds basic competency while cGMP certification develops expertise needed to implement and maintain state-of-the-art quality systems that drive competitive advantage
→ BPRHub's platform eliminates the complexity of cGMP implementation through integrated technology that automates compliance while providing real-time visibility into quality performance
FAQ
Q. What is the main difference between GMP and cGMP?
The main difference between GMP and cGMP lies in implementation sophistication and regulatory expectations. GMP provides basic quality standards through traditional documentation and manual processes, while cGMP demands contemporary implementation using current technology, risk-based approaches, and real-time monitoring systems. Current good manufacturing practices emphasize process understanding and continuous improvement rather than simple procedural compliance, requiring advanced quality systems that adapt to changing manufacturing conditions.
Q. What are the 5 P's of cGMP?
The 5 P's of cGMP are People, Premises, Processes, Products, and Procedures, as outlined in FDA guidance documents. People must be qualified and appropriately trained for their responsibilities. Premises must be designed, constructed, and maintained to facilitate good manufacturing operations. Processes must be validated and consistently controlled to ensure product quality. Products must be designed and developed according to current good manufacturing practices with appropriate quality attributes. Procedures must be written in clear, unambiguous language and followed systematically to ensure consistent implementation across all operations.
Q. What are the 4 main components of GMP?
The 4 main components of GMP include Personnel (qualified and trained staff), Premises (appropriate facilities and equipment), Procedures (documented systems and processes), and Products (quality specifications and controls). These components work together to create comprehensive quality systems that ensure consistent product quality while meeting regulatory requirements. Good manufacturing practices implementation requires systematic attention to all four components through integrated quality management approaches that address manufacturing operations holistically rather than as isolated elements.
Q. What are the 10 principles of cGMP?
The key cGMP requirements are established in 21 CFR Parts 210 and 211 and include: establishing written procedures for all operations, ensuring personnel qualifications and training, maintaining comprehensive documentation and records, designing and maintaining appropriate facilities, qualifying and calibrating all equipment, validating all manufacturing processes, implementing robust quality control systems, conducting stability studies for product shelf life, establishing complaint handling and investigation procedures, and maintaining recall capabilities for product retrieval. These requirements create comprehensive quality frameworks that ensure current good manufacturing practices implementation while supporting continuous improvement and regulatory compliance across all manufacturing activities.
Get insights that help you minimize risks and maximize profits.
Dive deeper into manufacturing compliance with our free resources.
We get it, compliance can get tough.
Here are some additional resources to help.
We get it, compliance can get tough. Here are some additional resources to help.
Get updates in your inbox

.svg)
%20(1).svg)





%20(1).svg)

.avif)

